Details of the Drug
General Information of Drug (ID: DMTY169)
| Drug Name |
Norethindrone
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Activella; Anhydrohydroxynorprogesterone; Anovulatorio; Anovule; Brevicon; Brevinor; Camila; Ciclovulan; Conceplan;Conludaf; Conludag; Demulen; Errin; Estrinor; Ethinylnortestosterone; Ethynylmortestosterone; Ethynylnortestosterone; Gencept; Genora; Gestest; Jenest; Loestrin; Menzol; Microneth; Micronett; Micronor; Micronovum; Milli; Minovlar; Nelova; Nodiol; Noraethisteronum; Noralutin; Norcept; Norcolut; Norcolute; Noresthisterone; Norethadrone; Norethin; Norethindirone; Norethisteron; Norethisterone; Norethisteronum; Norethyndron; Norethynodron; Norethynodrone; Noretisterona; Noretisterone; Norfor; Norgestin; Noriday; Norluten; Norlutin; Norluton; Normapause; Norpregneninlone; Norpregneninolone; Norpregneninotone; Orlest; Proluteasi; Synphase; Triella; Utovlan; Utovlar; Component of Noriday; Norethindrone Norethisterone; Norethindrone [USAN]; Norethisterone [Progestins]; Noretisterone [DCIT]; Primolut N; Brevinor 21; Brevinor 28; Noriday 28; Ortho 1 35; Ortho 7 7 7; Ovysmen 1 35; SC 4640; Synphasic 28; Trinovum 21; Brevinor-1 21; Brevinor-1 28; Camila (TN); Jenest-28; Micronor (TN); Mini-Pe; Mini-pill; Nor-QD; Nora-BE; Norcept-E; Norethin 1/35 E; Norethin 1/50 M; Norethindrone (USP); Norethisterone (JP15); Norethisteronum [INN-Latin]; Noretisterona [INN-Spanish]; Ortho-Novum 1 35; Ortho-Novum 1 50; Ortho-Novum 7 7 7; Ovysmen 0.5 35; Primolut-N; Tri-Norinyl; Ethinyl-19-nortestosterone; Nor-Q.D; Primolut-N (TN); Nor-Q.D.; 17-Ethinyl-19-nortestosterone; 17-Ethynyl-17-hydroxyestr-4-en-3-one; 17-alpha-Ethynyl-19-nortestosterone; 17-alpha-Ethynyl-4-estren-17-ol-3-one; 17-ethynyl-17beta-hydroxyestr-4-en-3-one; 17.alpha.-Ethinyl-19-nortestosterone; 17.alpha.-Ethynyl-19-nortestosterone; 17.alpha.-Ethynyl-4-estren-17-ol-3-one; 17alpha-Ethinyl-19-nortestosterone; 17alpha-Ethynyl-19-nortestosterone; 17alpha-Ethynyl-4-estren-17-ol-3-one; 19-Nor-17-alpha-ethynyltestosterone; 19-Nor-17-ethinyltestosterone; 19-Nor-17.alpha.-ethynyltestosterone; 19-Nor-17alpa-ethynyltestosterone; 19-Nor-17alpha-ethynyltestosterone; 19-Nor-ethindrone; 19-Nor-ethinyl-4,5-testosterone; 19-Norethindrone; 19-Norethinyltestosterone; 19-Norethisterone; 4-Estren-17alpha-ethynyl-17beta-ol-3-one
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Contraceptive Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
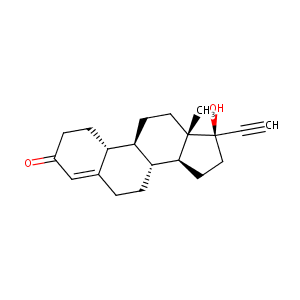 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 298.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Norethindrone
Coadministration of a Drug Treating the Disease Different from Norethindrone (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Norethindrone FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Progesterone receptor ligand binding pocket flexibility: crystal structures of the norethindrone and mometasone furoate complexes. J Med Chem. 2004 Jun 17;47(13):3381-7. | ||||
| 7 | Progestins as inhibitors of the human 20-ketosteroid reductases, AKR1C1 and AKR1C3. Chem Biol Interact. 2011 May 30;191(1-3):227-33. | ||||
| 8 | P-glycoprotein (P-gp/MDR1)-mediated efflux of sex-steroid hormones and modulation of P-gp expression in vitro. Pharm Res. 2004 Jul;21(7):1284-93. | ||||
| 9 | Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 2010 Dec; 118(2):485-500. | ||||
| 10 | The estrogenic activity of synthetic progestins used in oral contraceptives enhances fatty acid synthase-dependent breast cancer cell proliferation and survival. Int J Oncol. 2005 Jun;26(6):1507-15. | ||||
| 11 | Novel genotoxicity assays identify norethindrone to activate p53 and phosphorylate H2AX. Carcinogenesis. 2005 Oct;26(10):1811-20. doi: 10.1093/carcin/bgi132. Epub 2005 May 19. | ||||
| 12 | Pharmacologic reductions of total tau levels; implications for the role of microtubule dynamics in regulating tau expression. Mol Neurodegener. 2006 Jul 26;1:6. doi: 10.1186/1750-1326-1-6. | ||||
| 13 | A Gene Expression Biomarker Identifies Chemical Modulators of Estrogen Receptor in an MCF-7 Microarray Compendium. Chem Res Toxicol. 2021 Feb 15;34(2):313-329. doi: 10.1021/acs.chemrestox.0c00243. Epub 2021 Jan 6. | ||||
| 14 | Picomolar norethindrone in vitro stimulates the cell proliferation and activity of a human osteosarcoma cell line and increases bone collagen synthesis without an effect on bone resorption. J Bone Miner Res. 1994 May;9(5):695-703. doi: 10.1002/jbmr.5650090515. | ||||
| 15 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 16 | Faculty of Sexual & Reproductive Healthcare "FSRH Clinical Guidance: Drug Interactions with Hormonal Contraception.". | ||||
| 17 | Bailey DG, Arnold JMO, Spence JD "Grapefruit juice and drugs - how significant is the interaction." Clin Pharmacokinet 26 (1994): 91-8. [PMID: 8162660] | ||||
| 18 | Loi CM, Stern R, Koup JR, Vassos AB, Knowlton P, Sedman AJ "Effect of troglitazone on the pharmacokinetics of an oral contraceptive agent." J Clin Pharmacol 39 (1999): 410-7. [PMID: 10197300] | ||||
| 19 | Gunston GD, Mehta U "Potentially serious drug interactions with grapefruit juice." S Afr Med J 90 (2000): 41. [PMID: 10721388] | ||||
| 20 | Back DJ, Breckenridge AM, Crawford FE, MacIver M, Orne ML, Rowe PH "Interindividual variation and drug interactions with hormonal steroid contraceptives." Drugs 21 (1981): 46-61. [PMID: 7009137] | ||||
| 21 | Product Information. Cosentyx (secukinumab). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 22 | Product Information. Ambien (zolpidem). sanofi-aventis, Bridgewater, NJ. | ||||
| 23 | Lazar JD, Wilner KD "Drug interactions with fluconazole." Rev Infect Dis 12 Suppl 3 (1990): s327-33. [PMID: 2330488] | ||||
| 24 | Gardner MJ, Tornatore KM, Jusko WJ, Kanarkowski R "Effects of tobacco smoking and oral contraceptive use on theophylline disposition." Br J Clin Pharmacol 16 (1983): 271-80. [PMID: 6626419] | ||||
| 25 | Product Information. Synercid (dalfopristin-quinupristin) Rhone-Poulenc Rorer, Collegeville, PA. | ||||
| 26 | Product Information. Ketek (telithromycin). Aventis Pharmaceuticals, Bridgewater, NJ. | ||||
| 27 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 28 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 29 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 30 | Product Information. Lipitor (atorvastatin). Parke-Davis, Morris Plains, NJ. | ||||
| 31 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 32 | Ito D, Amano T, Sato H, Fukuuchi Y "Paroxysmal hypertensive crises induced by selegiline in a patient with Parkinson's disease." J Neurol 248 (2001): 533-4. [PMID: 11499649] | ||||
| 33 | Back DJ, Grimmer SF, Orme ML, Proudlove D, Mann RD, Breckenridge AM "Evaluation of Committee on Safety of Medicines yellow card reports on oral contraceptive-drug interactions with anticonvulsants and antibiotics." Br J Clin Pharmacol 25 (1988): 527-32. [PMID: 3408633] | ||||
| 34 | EMEA. European Medicines Agency "EPARs. European Union Public Assessment Reports.". | ||||
| 35 | Product Information. Briviact (brivaracetam). UCB Pharma Inc, Smyrna, GA. | ||||
| 36 | Back DJ, Breckenridge AM, Crawford F, et al. "The effect of rifampicin on norethisterone pharmacokinetics." Eur J Clin Pharmacol 15 (1979): 193-7. [PMID: 37091] | ||||
| 37 | Product Information. Prezista (darunavir). Ortho Biotech Inc, Bridgewater, NJ. | ||||
| 38 | Product Information. Orladeyo (berotralstat). BioCryst Pharmaceuticals Inc, Durham, NC. | ||||
| 39 | Product Information. Retevmo (selpercatinib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 40 | Product Information. Copiktra (duvelisib). Verastem, Inc., Needham, MA. | ||||
| 41 | Product Information. Tasigna (nilotinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 42 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 43 | Product Information. Xeglyze (abametapir topical). Dr. Reddy's Laboratories Inc, Upper Saddle River, NJ. | ||||
| 44 | Product Information. Zokinvy (lonafarnib). Eiger BioPharmaceuticals, Palo Alto, CA. | ||||
| 45 | Product Information. Prograf (tacrolimus). Fujisawa, Deerfield, IL. | ||||
| 46 | Product Information. Tavalisse (fostamatinib). Rigel Pharmaceuticals, South San Francisco, CA. | ||||
