| 1 |
ClinicalTrials.gov (NCT01065662) AZD2171 and Temsirolimus in Patients With Advanced Gynecological Malignancies
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5892).
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5664).
|
| 4 |
Advances in kinase targeting: current clinical use and clinical trials. Trends Pharmacol Sci. 2014 Nov;35(11):604-20.
|
| 5 |
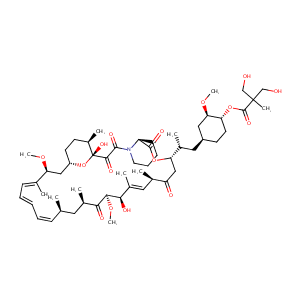
Association of NR1I2, CYP3A5 and ABCB1 genetic polymorphisms with variability of temsirolimus pharmacokinetics and toxicity in patients with metastatic bladder cancer. Cancer Chemother Pharmacol. 2017 Sep;80(3):653-659.
|
| 6 |
Drug Interactions Flockhart Table
|
| 7 |
A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22.
|
| 8 |
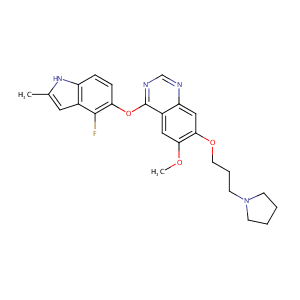
In vitro hepatic metabolism of cediranib, a potent vascular endothelial growth factor tyrosine kinase inhibitor: interspecies comparison and human enzymology. Drug Metab Dispos. 2010 Oct;38(10):1688-97.
|
|
|
|
|
|
|