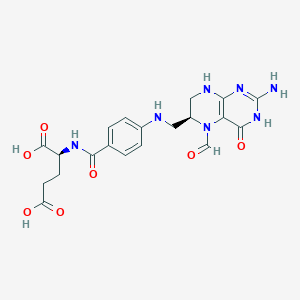
| Molecular Interaction Atlas (MIA) |
|
| Indication(s) of Pralatrexate |
| Disease Entry |
ICD 11 |
Status |
REF |
| Breast cancer |
2C60-2C65
|
Approved |
[2] |
| Peripheral T-cell lymphoma |
2A90.C
|
Approved |
[2] |
| Primary cutaneous peripheral T-cell lymphoma not otherwise specified |
N.A.
|
Approved |
[3] |
|
|
|
Pralatrexate Interacts with 1 DTT Molecule(s)
| DTT Name |
DTT ID |
UniProt ID |
Mode of Action |
REF |
|
Polypeptide deformylase (PDF)
|
TT9SL3Q
|
DEFM_HUMAN
|
Inhibitor
|
[6] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
|
Pralatrexate Interacts with 2 DTP Molecule(s)
| DTP Name |
DTP ID |
UniProt ID |
Mode of Action |
REF |
|
Folate transporter 1 (SLC19A1)
|
DTOSN46
|
S19A1_HUMAN
|
Substrate
|
[7] |
|
Proton-coupled folate transporter (SLC46A1)
|
DTDJEMI
|
PCFT_HUMAN
|
Substrate
|
[7] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
|
Pralatrexate Interacts with 1 DME Molecule(s)
| DME Name |
DME ID |
UniProt ID |
Mode of Action |
REF |
|
Folylpolyglutamate synthase (FPGS)
|
DECWT2V
|
FOLC_HUMAN
|
Metabolism
|
[8] |
| ------------------------------------------------------------------------------------ |
|
|
|
|
|
| Indication(s) of Levoleucovorin |
| Disease Entry |
ICD 11 |
Status |
REF |
| Anemia |
3A00-3A9Z
|
Approved |
[4] |
| Colon adenocarcinoma |
N.A.
|
Approved |
[5] |
| Colorectal carcinoma |
N.A.
|
Approved |
[5] |
| Non-small-cell lung cancer |
2C25.Y
|
Approved |
[5] |
| Pervasive developmental disorder |
6A00-6A0Z
|
Approved |
[5] |
| Pneumocystis pneumonia |
CA40.20
|
Approved |
[5] |
| Rectal adenocarcinoma |
2B92
|
Approved |
[5] |
| Rectum mucinous adenocarcinoma |
N.A.
|
Approved |
[5] |
| Colon cancer |
2B90.Z
|
Investigative |
[5] |
| Colon mucinous adenocarcinoma |
N.A.
|
Investigative |
[5] |
| Gastric cancer |
2B72
|
Investigative |
[5] |
| Megaloblastic anemia |
N.A.
|
Investigative |
[5] |
| Methotrexate toxicity |
N.A.
|
Investigative |
[5] |
|
|
|
|
|
|
|
|
|