| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6693).
|
| 3 |
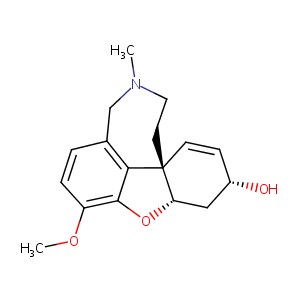
Galantamine FDA Label
|
| 4 |
Tetracycline FDA Label
|
| 5 |
How many modes of action should an antibiotic have Curr Opin Pharmacol. 2008 Oct;8(5):564-73.
|
| 6 |
[From symptomatic to disease modifying therapy Recent developments in the pharmacotherapy of Alzheimer's disease]. Fortschr Neurol Psychiatr. 2009 Jun;77(6):326-33.
|
| 7 |
Clinical pharmacokinetics of galantamine. Clin Pharmacokinet. 2003;42(15):1383-92.
|
| 8 |
Lichens of parmelioid clade as promising multitarget neuroprotective agents. Chem Res Toxicol. 2019 Jun 17;32(6):1165-1177.
|
| 9 |
Inhibition of human carboxylesterases hCE1 and hiCE by cholinesterase inhibitors. Chem Biol Interact. 2013 Mar 25;203(1):226-30.
|
| 10 |
Cholinergic drugs potentiate human nicotinic alpha4beta2 acetylcholine receptors by a competitive mechanism. Eur J Pharmacol. 2005 Feb 21;509(2-3):97-108. doi: 10.1016/j.ejphar.2004.12.037.
|
| 11 |
Potencies and selectivities of inhibitors of acetylcholinesterase and its molecular forms in normal and Alzheimer's disease brain. Acta Biol Hung. 2003;54(2):183-9. doi: 10.1556/ABiol.54.2003.2.7.
|
| 12 |
Determination of phospholipidosis potential based on gene expression analysis in HepG2 cells. Toxicol Sci. 2007 Mar;96(1):101-14.
|
| 13 |
The glycylcyclines: a comparative review with the tetracyclines. Drugs. 2004;64(1):63-88.
|
| 14 |
Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64.
|
| 15 |
Arginine-482 is not essential for transport of antibiotics, primary bile acids and unconjugated sterols by the human breast cancer resistance protein (ABCG2). Biochem J. 2005 Jan 15;385(Pt 2):419-26.
|
| 16 |
Human organic anion transporters mediate the transport of tetracycline. Jpn J Pharmacol. 2002 Jan;88(1):69-76.
|
| 17 |
Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol. 2005 May;57(5):573-8.
|
| 18 |
Inhibition of glutathione S-transferases by antimalarial drugs possible implications for circumventing anticancer drug resistance. Int J Cancer. 2002 Feb 10;97(5):700-5.
|
| 19 |
A comprehensive in vitro and in silico analysis of antibiotics that activate pregnane X receptor and induce CYP3A4 in liver and intestine. Drug Metab Dispos. 2008 Aug;36(8):1689-97.
|
| 20 |
Tetracycline affects abnormal properties of synthetic PrP peptides and PrP(Sc) in vitro. J Mol Biol. 2000 Jul 28;300(5):1309-22. doi: 10.1006/jmbi.2000.3840.
|
| 21 |
Effects of residual levels of tetracycline on the barrier functions of human intestinal epithelial cells. Food Chem Toxicol. 2017 Nov;109(Pt 1):253-263. doi: 10.1016/j.fct.2017.09.004. Epub 2017 Sep 4.
|
| 22 |
Synthesis and in vitro evaluation of targeted tetracycline derivatives: effects on inhibition of matrix metalloproteinases. Bioorg Med Chem. 2007 Mar 15;15(6):2368-74. doi: 10.1016/j.bmc.2007.01.026. Epub 2007 Jan 19.
|
| 23 |
Advantageous use of HepaRG cells for the screening and mechanistic study of drug-induced steatosis. Toxicol Appl Pharmacol. 2016 Jul 1;302:1-9. doi: 10.1016/j.taap.2016.04.007. Epub 2016 Apr 16.
|
| 24 |
Old drug, new target: ellipticines selectively inhibit RNA polymerase I transcription. J Biol Chem. 2013 Feb 15;288(7):4567-82. doi: 10.1074/jbc.M112.411611. Epub 2013 Jan 4.
|
| 25 |
Increased hepatic Fatty Acid uptake and esterification contribute to tetracycline-induced steatosis in mice. Toxicol Sci. 2015 Jun;145(2):273-82. doi: 10.1093/toxsci/kfv049. Epub 2015 Mar 4.
|
| 26 |
Effect of common medications on the expression of SARS-CoV-2 entry receptors in liver tissue. Arch Toxicol. 2020 Dec;94(12):4037-4041. doi: 10.1007/s00204-020-02869-1. Epub 2020 Aug 17.
|
| 27 |
Multichannel liquid chromatography-tandem mass spectrometry cocktail method for comprehensive substrate characterization of multidrug resistance-associated protein 4 transporter. Pharm Res. 2007 Dec;24(12):2281-96.
|
|
|
|
|
|
|