Details of the Drug Combinations
General Information of This Drug (ID: DM3FXMA)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Nalone; Nalossone; Naloxona; Naloxonum; Narcan; Narcanti; Narcon; DBL Naloxone; Nalossone [DCIT]; Naloxone HCl; EN 1530base; DBL Naloxone (TN); L-Naloxone; N-Allylnoroxymorphone; Nalone (TN); Naloxona [INN-Spanish]; Naloxone (INN); Naloxone [INN:BAN]; Naloxonum [INN-Latin]; Narcan (TN); Narcanti (TN); N-Allyl-noroxymorphone; L-N-Allyl-14-hydroxynordihydromorphinone; L-N-Allyl-7,8-dihydro-14-hydroxynormorphinone; N-Allyl-4,5alpha-epoxy-3,14-dihydroxy-6-morphinanon; Morphinan-6-one, 17-allyl-4,5alpha-epoxy-3,14-dihydroxy-(8CI); Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-(2-propenyl)-, (5alpha)-(9CI); (-)-N-allyl-14-hydroxynordihydroxymorphinan-6-one; (-)-Naloxone; (5alpha)-3,14-dihydroxy-17-prop-2-en-1-yl-4,5-epoxymorphinan-6-one; (5alpha)-4,5-Epoxy-3,14-dihydroxy-17-(2-propenyl)morphinan-6-one; 1-N-Allyl-14-hydroxynordihydromorphinone; 1-N-Allyl-7,8-dihydro-14-hydroxynormorphinone; 12-Allyl-7,7a,8,9-tetrahydro-3,7a-dihydroxy-4aH-8,9c-iminoethanophenanthro(4,5-bcd)furanone; 12-Allyl-7,7a,8,9-tetrahydro-3,7a-dihydroxy-4aH-8,9c-iminoethanophenanthro[4,5-bcd]furan-5(6H)-one; 17-Allyl-4,5-alpha-epoxy-3,14-dihydroxymorphinan-6-one; 17-Allyl-4,5alpha-epoxy-3,14-dihydroxymorphinan-6-one; 17-allyl-3,14-dihydroxy-4,5alpha-epoxymorphinan-6-one; 3,14-dihydroxy-17-(prop-2-en-1-yl)-4,5alpha-epoxymorphinan-6-one
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antinarcotic Agents
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
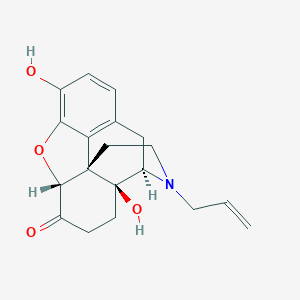 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
6 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
1 Approved Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
