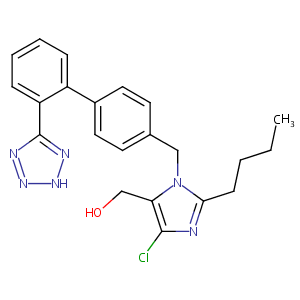
| 1 |
Losartan FDA Label
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 590).
|
| 3 |
ClinicalTrials.gov (NCT04343001) Coronavirus Response - Active Support for Hospitalised Covid-19 Patients. U.S. National Institutes of Health.
|
| 4 |
ClinicalTrials.gov (NCT04815902) Use of Senolytic and Anti-Fibrotic Agents to Improve the Beneficial Effect of Bone Marrow Stem Cells for Osteoarthritis
|
| 5 |
ClinicalTrials.gov (NCT00732407) Aliskiren's Effect on Arterial Stiffness and Platelet Function in Patients With Diabetes Mellitus (DM)
|
| 6 |
ClinicalTrials.gov (NCT00151827) Olmesartan Medoxomil in Hypertension and Renal Impairment
|
| 7 |
ClinicalTrials.gov (NCT00555217) VA NEPHRON-D: Diabetes iN Nephropathy Study
|
| 8 |
ClinicalTrials.gov (NCT00446563) Efficacy and Safety of Valsartan in Combination With Amlodipine Compared to Losartan Plus Hydrochlorothiazide in Patients With Hypertension and Left Ventricular Hypertrophy
|
| 9 |
ClinicalTrials.gov (NCT02704494) Resveratrol's Effects in Diabetic Nephropathy
|
| 10 |
ClinicalTrials.gov (NCT02190318) Residual Renal Function Preservation in Peritoneal Dialysis Patients
|
| 11 |
Therapeutic approaches to diabetic nephropathy--beyond the RAS. Nat Rev Nephrol. 2014 Jun;10(6):325-46.
|
| 12 |
ClinicalTrials.gov (NCT00732966) Ocsaar and CYP2C9 Ploymorphism, Is There a Connection Between Pharmacokinetics, Pharmacodynamics and Pharmacogenetics?
|
| 13 |
ClinicalTrials.gov (NCT04447235) Early Treatment With Ivermectin and LosarTAN for Cancer Patients With COVID-19 Infection
|
| 14 |
ClinicalTrials.gov (NCT01821729) Proton w/FOLFIRINOX-Losartan for Pancreatic Cancer
|
| 15 |
ClinicalTrials.gov (NCT04074551) A Phase 3 Study to Compare the Efficacy and Safety of Co-administered HGP0608, HGP0904 and HCP1306 Versus HCP1701 in Patients With Hypertension and Dyslipidemia
|
| 16 |
ClinicalTrials.gov (NCT05619653) Myocardial Protection in Patients With Post-acute Inflammatory Cardiac Involvement Due to COVID-19
|
| 17 |
ClinicalTrials.gov (NCT01176032) ALiskiren or Losartan Effects on bioMARKers of Myocardial Remodeling
|
| 18 |
ClinicalTrials.gov (NCT04238702) Renohemodynamic Effects of Combined empagliflOzin and LosARtan
|
| 19 |
ClinicalTrials.gov (NCT03006952) Add-on Pentoxifylline to Losartan Versus Increasing Dose of Losartan on NT-PRO BNP in Type 2 Diabetics With Nephropathy
|
| 20 |
ClinicalTrials.gov (NCT02188121) Fixed Dose Intervention Trial of New England Enhancing Survival in SMI Patients
|
| 21 |
ClinicalTrials.gov (NCT03953950) Effect of Add-on Spironolactone to Losartan Versus Losartan Alone on Peritoneal Membrane Among Peritoneal Dialysis Patients
|
|
|
|
|
|
|