Details of the Drug Combinations
General Information of This Drug (ID: DMHVJFK)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cytotec; GyMiso; Isprelor; Misopess; Misoprostolum; Misotol; SC 29333; Cytotec (TN); Misoprostolum [INN-Latin]; SC-29333; XP-16J; Misoprostol (JAN/USAN/INN); Methyl (11alpha,13E)-11,16-dihydroxy-16-methyl-9-oxoprost-13-en-1-oate; Methyl (+-)-11-alpha,16-dihydroxy-16-methyl-9-oxoprost-13-en-1-oate; Methyl 7-[(1R,2R,3R)-3-hydroxy-2-[(E)-4-hydroxy-4-methyloct-1-enyl]-5-oxocyclopentyl]heptanoate; (+/-)-15-Deoxy-(16RS)-16-hydroxy-16-methylprostaglandin E1 methyl ester; (11-alpha,13E)-(+-)-11,16-Dihydroxy-16-methyl-9-oxoprost-13-en-1-oic acid methyl ester
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Abortifacient Agents
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
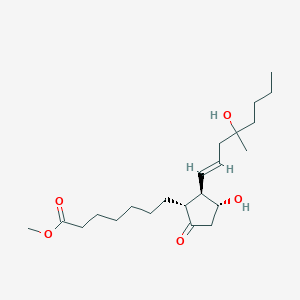 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
12 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
