Details of the Drug Combinations
General Information of This Drug (ID: DMKBJWP)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
CFF; Cafamil; Cafecon; Cafeina; Cafeine; Caffedrine; Caffein; Caffeina; Caffenium; Caffine; Cafipel; Coffein; Coffeine; Coffeinum; DHCplus; Dasin; Dexitac; Diurex; Durvitan; Enerjets; Ercatab; Guaranine; Hycomine; Kofein; Koffein; Mateina; Methyltheobromide; Methyltheobromine; Miudol; Nodaca; Organex; Percutafeine; Phensal; Stim; Teina; Thein; Theine; Tirend; Vivarin; Anacin Maximum Strength; Anhydrous caffeine; Caffedrine Caplets; Caffeina [Italian]; Caffeine Pure; Caffeine solution; Coffein [German]; Coffeinum N; Coffeinum Purrum; Component of Cafergot; DHC Plus; Dexitac Stay Alert Stimulant; Eldiatric C; GlaxoSmithKline Brand of Caffeine; Hycomine Compound; Keep Alert; Kofein [Czech]; Koffein [German]; Merck dura Brand of Caffeine; Methylxanthine theophylline; Midol Maximum Strength; Monomethyl derivative of Theophylline; Natural Caffeinum; Nix Nap; No Doz; Nodoz Maximum Strength Caplets; Passauer Brand of Caffeine; Percoffedrinol N; Pierre Fabre Brand of Caffeine; Quick Pep; Republic Drug Brand of Caffeine; Seid Brand of Caffeine; Theobromine Me; Theophylline Me; C 0750; Propoxyphene Compound 65; SK 65 Compound; TNP00310; Thompson Brand 1 of Caffeine; Thompson Brand 2 of Caffeine; Alert-pep; Anhydrous caffeine (JP15); Anhydrous caffeine (TN); Berlin-Chemie Brand of Caffeine; Bristol-Myers Squibb Brand of Caffeine; Cafcit (TN); Caffeine (USP); Caffeine (natural); Caffeine [BAN:JAN]; Caffeine, Monohydrate; Caffeine, anhydrous; Caffeine, synthetic; No-Doz; Pep-Back; Propoxyphene Compound-65; Quick-Pep; Refresh'n; SK-65 Compound; Tri-Aqua; Ultra Pep-Back; Wake-Up; CU-01000012617-3; P-A-C Analgesic Tablets; Theophylline, 7-methyl; Xanthine, 1,3,7-trimethyl; 1,3,7-Trimethyl-2,6-dioxopurine; 1,3,7-Trimethylpurine-2,6-dione; 1,3,7-Trimethylxanthine; 1-3-7-TRIMETHYLXANTHINE; 1-methyltheobromine; 3,7-dihydro-1,3,7-trimethyl-1H-purine; 7-Methyltheophylline
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antihypertensive Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
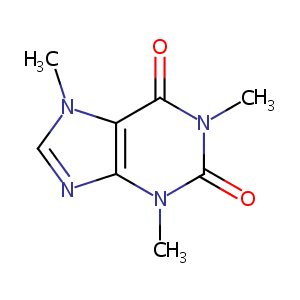 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
13 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
