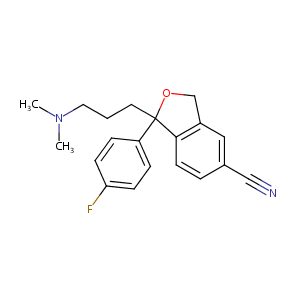
| Synonyms |
Akarin; Celapram; Celexa; Celius; Ciazil; Cilift; Cipram; Ciprapine; Citabax; Citadur; Citalec; Citalopramum; Citopam; Cytalopram; Dalsan; Humorup; Nitalapram; Oropram; Pramcit; Recital; Seropram; Talam; Talohexal; Temperax; Vodelax; Zentius; Zetalo; Citalopram [Celexa]; [3H]Citalopram; Akarin (TN); Celapram (TN); Celexa (TN); Celius (TN); Ciazil (TN); Cilift (TN); Cipram (TN); Cipramil (TN); Ciprapine (TN); Citabax (TN); Citadur (TN); Citalec (TN); Citalopram [INN:BAN]; Citalopramum [INN-Latin]; Citaxin (TN); Citol (TN); Citopam (TN); Citox (TN); Citrol (TN); Dalsan (TN); Lu 10-171; Recital (TN); Seropram (TN); Talam (TN); Zentius (TN); Zetalo (TN); AE-641/00603021; Citalopram (USP/INN); Lu-10-171; 1,3-Dihydro-1-(3-(dimethylamino)propyl)-1-(4-fluorophenyl)-5-isobenzofurancarbonitrile; 1,3-dihydro[3,4]benzofuran-5-carbonitrile; 1-(3-(Dimethylamino)propyl)-1-(p-fluorophenyl)-5-phthalancarbonitrile; 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-2-benzofuran-5-carbonitrile; 1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3H-2-benzofuran-5-carbonitrile
|