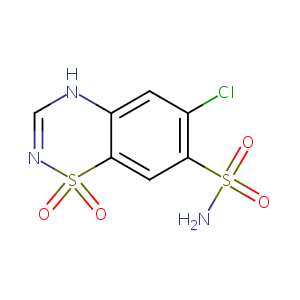
| Synonyms |
Aldoclor; Alurene; Chloriazid; Chlorosal; Chlorothiazid; Chlorothiazidum; Chlorotiazida; Chlorthiazid; Chlorthiazide; Chlorthiazidum; Chlortiazid; Chlorurit; Chlotride; Chlrosal; Clorotiazida; Clorotiazide; Clotride; Diupres; Diuresal; Diuril; Diurilix; Diurite; Diutrid; Flumen; Minzil; Salisan; Salunil; Saluretil; Saluric; Thiazide; Urinex; Warduzide; Yadalan; Clorotiazide [DCIT]; Component of Aldoclor; Diuril Boluses; C 4911; Chlorothiazidum [INN-Latin]; Clorotiazida [INN-Spanish]; Diuril (TN); Diuril Boluses, Veterinary; Neo-Dema; Sk-chlorothiazide; Chlorothiazide [USAN:INN:BAN]; Diuril, Chlotride, Chlorothiazide; Chlorothiazide (JAN/USP/INN); 2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-, 1,1-dioxide; 6-Chloro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 6-Chloro-7-sulfamoyl-2H-1,2,4-benzothiadiazine 1,1-dioxide; 6-chloro-4H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
|