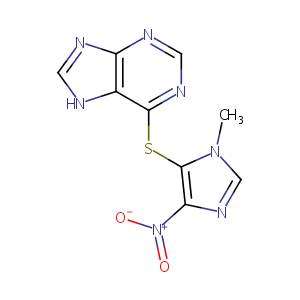
| Synonyms |
Azamun; Azanin; Azasan; Azathioprin; Azathioprinum; Azathiopurine; Azatioprin; Azatioprina; Azothioprine; Ccucol; Cytostatics; Immuran; Imuran; Imurek; Imurel; Methylnitroimidazolylmercaptopurine; Muran; Azamun [Czech]; Azathioprine sodium; Azatiopr in; A 4638; BW 57322; Azamun (TN); Azasan (TN); Azathioprinum [INN-Latin]; Azatioprina [INN-Spanish]; BW 57-322; Imuran (TN); Imurel (TN); [Methyl(nitroimidazolyl)mercaptopurine]; AI-981/34845012; BW-57-322; Azathioprine (JP15/USP/INN); Azathioprine [USAN:INN:BAN:JAN]; B. W. 57-322; Thiopurine 6-(1-methyl-4-nitroimidazol-5-yl); Azasan, Imuran, Azamun, BW-57-322, NSC-39084, Azathioprine; 6-((1-Methyl-4-nitro-1H-imidazol-5-yl)thio)-1H-purine; 6-((1-Methyl-4-nitroimidazol-5-yl)thio)purine; 6-(1'-Methyl-4'-nitro-5'-imidazolyl)-mercaptopurine; 6-(1'-Methyl-4'-nitro-5'-imidazolyl)mercaptopurine; 6-(1-Methyl-4-nitroimidazol-5-yl)thiopurine; 6-(1-Methyl-4-nitroimidazol-5-ylthio)purin; 6-(1-Methyl-4-nitroimidazol-5-ylthio)purin [Czech]; 6-(1-Methyl-p-nitro-5-imidazolyl)-thiopurine; 6-(1-Methyl-p-nitro-5-imidazolyl)thiopurine; 6-(3-Methyl-5-nitro-3H-imidazol-4-ylsulfanyl)-7H-purine; 6-(3-methyl-5-nitroimidazol-4-yl)sulfanyl-7H-purine; 6-(Methyl-p-nitro-5-imidazolyl)-thiopurine; 6-(Methyl-p-nitro-5-imidazolyl)thiopurine; 6-({4-nitro-1-methyl-1H-imidazol-5-yl}sulfanyl)-7H-purine; 6-1'-Methyl,4'-nitro,5'-imidazolyl mercaptopurine; 6-[(1-Methyl-4-nitroimidazol-5-yl)-thio] purine; 6-[(1-Methyl-4-nitroimidazol-5-yl)thio]purine; 6-[(1-methyl-4-nitro-1H-imidazol-5-yl)sulfanyl]-7H-purine; 6-[(1-methyl-4-nitro-1H-imidazol-5-yl)thio]-1H-Purine; 6-[(1-methyl-4-nitro-1H-imidazol-5-yl)thio]-7H-purine; 6-[(1-methyl-4-nitro-1H-imidazol-5-yl)thio]-9H-purine
|