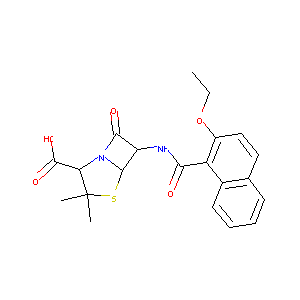
| Synonyms |
Nafcilina; Nafcilline; Nafcillinum; Nallpen; Naphcillin; Naphthamidopenicillin; Unipen; NAFCILLIN SODIUM; Nafcillin sodium for injection; Nallpen In Plastic Container; Nafcilin-1; Nafcilina [INN-Spanish]; Nafcillin & VRC3375; Nafcillin (INN); Nafcillin [INN:BAN]; Nafcilline [INN-French]; Nafcillinum [INN-Latin]; Nafcillin, Monosodium Salt, Anhydrous; (2-Ethoxy-1-naphthyl)penicillin; (2-ethoxy-1-naphthalenyl)penicillin; (2S,5R,6R)-6-({[2-(ethyloxy)naphthalen-1-yl]carbonyl}amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-[(2-ethoxynaphthalene-1-carbonyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-{[(2-ethoxynaphthalen-1-yl)carbonyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 6-(2-ethoxy-1-naphthamido)penicillanic acid; 6-[(2-Ethoxy-naphthalene-1-carbonyl)-amino]-3,3-dimethyl-7-oxo-4-thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylic acid anion; 6beta-(2-ethoxynaphthalene-1-carboxamido)-2,2-dimethylpenam-3alpha-carboxylic acid
|