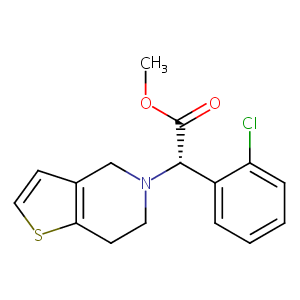
| Synonyms |
clopidogrel; 113665-84-2; (S)-Clopidogrel; Plavix; Clopidogrel [INN:BAN]; (+)-(S)-Clopidogrel; (+)-Clopidogrel; UNII-A74586SNO7; HSDB 7430; CLOPIDOGREL SULFATE; CHEBI:37941; GKTWGGQPFAXNFI-HNNXBMFYSA-N; A74586SNO7; CPD000550475; Zyllt; Clopidogrel (TN); SR-25990C; Plavix (TN); methyl (2S)-(2-chlorophenyl)(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)ethanoate; Clopidogrelum; Thrombo; Clopidogrel BMS; Clopidogrel Hexal; Clopidogrel Acino; methyl (2S)-2-(2-chlorophenyl)-2-{4H,5H,6H,7H-thieno[3,2-c]pyridin-5-yl}acetate; PM-103; Clopidogrel (captisol-enabled); Clopidogrel (captisol-enabled), The Medicines Co; Clopidogrel (captisol-enabled, cardiovascular disease), Cydex; Clopidogrel (captisol-enabled, cardiovascular disease), Prism Pharmaceuticals
|