Details of the Drug
General Information of Drug (ID: DMOL54H)
| Drug Name |
Clopidogrel
|
|||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
clopidogrel; 113665-84-2; (S)-Clopidogrel; Plavix; Clopidogrel [INN:BAN]; (+)-(S)-Clopidogrel; (+)-Clopidogrel; UNII-A74586SNO7; HSDB 7430; CLOPIDOGREL SULFATE; CHEBI:37941; GKTWGGQPFAXNFI-HNNXBMFYSA-N; A74586SNO7; CPD000550475; Zyllt; Clopidogrel (TN); SR-25990C; Plavix (TN); methyl (2S)-(2-chlorophenyl)(6,7-dihydrothieno[3,2-c]pyridin-5(4H)-yl)ethanoate; Clopidogrelum; Thrombo; Clopidogrel BMS; Clopidogrel Hexal; Clopidogrel Acino; methyl (2S)-2-(2-chlorophenyl)-2-{4H,5H,6H,7H-thieno[3,2-c]pyridin-5-yl}acetate; PM-103; Clopidogrel (captisol-enabled); Clopidogrel (captisol-enabled), The Medicines Co; Clopidogrel (captisol-enabled, cardiovascular disease), Cydex; Clopidogrel (captisol-enabled, cardiovascular disease), Prism Pharmaceuticals
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
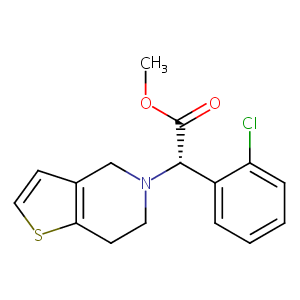 |
|||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 321.8 | ||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.8 | |||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | |||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||
| ADMET Property |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Same Disease as Clopidogrel
Coadministration of a Drug Treating the Disease Different from Clopidogrel (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Clopidogrel FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7150). | ||||
| 3 | Preventing Cardiac Complication of COVID-19 Disease With Early Acute Coronary Syndrome Therapy: A Randomised Controlled Trial. (C-19-ACS) | ||||
| 4 | BDDCS applied to over 900 drugs | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013 Sep;94(3):317-23. doi: 10.1038/clpt.2013.105. Epub 2013 May 22. | ||||
| 8 | P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med. 2003 May;3(2):113-22. | ||||
| 9 | Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006 Nov;80(5):486-501. | ||||
| 10 | Substrates, inducers, inhibitors and structure-activity relationships of human Cytochrome P450 2C9 and implications in drug development. Curr Med Chem. 2009;16(27):3480-675. | ||||
| 11 | Cytochrome P450 3A inhibition by ketoconazole affects prasugrel and clopidogrel pharmacokinetics and pharmacodynamics differently. Clin Pharmacol Ther. 2007 May;81(5):735-41. | ||||
| 12 | Impact of the CYP2C19 gene polymorphism on clopidogrel personalized drug regimen and the clinical outcomes. Clin Lab. 2016 Sep 1;62(9):1773-1780. | ||||
| 13 | Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin Pharmacokinet. 2015 Feb;54(2):147-66. | ||||
| 14 | Clopidogrel pathway. Pharmacogenet Genomics. 2010 Jul;20(7):463-5. | ||||
| 15 | Differential effect of clopidogrel and aspirin on the release of BDNF from platelets. J Neuroimmunol. 2011 Sep 15;238(1-2):104-6. doi: 10.1016/j.jneuroim.2011.06.015. Epub 2011 Jul 31. | ||||
| 16 | Clopidogrel increases expression of chemokines in peripheral blood mononuclear cells in patients with coronary artery disease: results of a double-blind placebo-controlled study. J Thromb Haemost. 2006 Oct;4(10):2140-7. doi: 10.1111/j.1538-7836.2006.02131.x. Epub 2006 Jul 17. | ||||
| 17 | Angiogenesis inhibitor SR 25989 upregulates thrombospondin-1 expression in human vascular endothelial cells and foreskin fibroblasts. Biol Cell. 1997 Jul;89(4):295-307. | ||||
| 18 | Association of CYP1A1 and CYP1B1 inhibition in in vitro assays with drug-induced liver injury. J Toxicol Sci. 2021;46(4):167-176. doi: 10.2131/jts.46.167. | ||||
| 19 | Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010 Jan;38(1):92-9. doi: 10.1124/dmd.109.029132. | ||||
| 20 | Identification of novel agonists by high-throughput screening and molecular modelling of human constitutive androstane receptor isoform 3. Arch Toxicol. 2019 Aug;93(8):2247-2264. doi: 10.1007/s00204-019-02495-6. Epub 2019 Jul 16. | ||||
| 21 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 22 | Canadian Pharmacists Association. | ||||
| 23 | Itkonen MK, Tornio A, Neuvonen M, Neuvonen PJ, Niemi M, Backman JT "Clopidogrel Markedly Increases the Plasma Concentrations of the CYP2C8 Substrate Pioglitazone." Drug Metab Dispos (2016):. [PMID: 27260150] | ||||
| 24 | Agencia Espaola de Medicamentos y Productos Sanitarios Healthcare "Centro de informacion online de medicamentos de la AEMPS - CIMA.". | ||||
| 25 | Clarke TA, Waskell LA "The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin." Drug Metab Dispos 31 (2003): 53-9. [PMID: 12485953] | ||||
| 26 | Collet JP, Hulot JS, Pena A, et al. "Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study." Lancet 373 (2009): 309-17. [PMID: 19108880] | ||||
| 27 | Eggleston W, Clark KH, Marraffa JM "Loperamide abuse associated with cardiac dysrhythmia and death." Ann Emerg Med 69 (2017): 83-6. [PMID: 27140747] | ||||
| 28 | Product Information. Tykerb (lapatinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 29 | Product Information. Tukysa (tucatinib). Seattle Genetics Inc, Bothell, WA. | ||||
| 30 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 31 | Alderman CP, Moritz CK, Ben-Tovim DI "Abnormal platelet aggregation associated with fluoxetine therapy." Ann Pharmacother 26 (1992): 1517-9. [PMID: 1482806] | ||||
| 32 | Anand S, Yusuf S, Xie C, et al. "Oral anticoagulant and antiplatelet therapy and peripheral arterial disease." N Engl J Med 357 (2007): 217-27. [PMID: 17634457] | ||||
| 33 | Product Information. Ofev (nintedanib). Boehringer Ingelheim, Ridgefield, CT. | ||||
| 34 | Price AJ, Frcpath DO "Is there a clinical interaction between low molecular weight heparin and non-steroidal analgesics after total hip replacement?" Ann R Coll Surg Engl 77 (1995): 395. [PMID: 7486773] | ||||
| 35 | Product Information. Xarelto (rivaroxaban). Bayer Inc, Toronto, IA. | ||||
| 36 | Product Information. Panhematin (hemin). Recordati Rare Diseases Inc, Lebanon, NJ. | ||||
| 37 | Heck AM, DeWitt BA, Lukes AL "Potential interactions between alternative therapies and warfarin." Am J Health Syst Pharm 57 (2000): 1221-7 quiz 1228-30. [PMID: 10902065] | ||||
| 38 | Product Information. Plavix (clopidogrel). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 39 | Product Information. Acular (ketorolac). Allergan Inc, Irvine, CA. | ||||
| 40 | Product Information. Dexilant (dexlansoprazole). Takeda Pharmaceuticals America, Lincolnshire, IL. | ||||
| 41 | Product Information. Aptivus (tipranavir). Boehringer-Ingelheim, Ridgefield, CT. | ||||
| 42 | Product Information. Viberzi (eluxadoline). Actavis Pharma, Inc., Parsippany, NJ. | ||||
| 43 | Caruso V, Iacoviello L, Di Castelnuovo A, et.al "Thrombotic complications in childhood acute lymphoblastic leukemia: a meta-analysis of 17 prospective studies comprising 1752 pediatric patients." Blood 108 (2006): 2216-22. [PMID: 16804111] | ||||
| 44 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 45 | Product Information. Iclusig (ponatinib). Ariad Pharmaceuticals Inc, Cambridge, MA. | ||||
| 46 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 47 | Product Information. Brukinsa (zanubrutinib). BeiGene USA, Inc, San Mateo, CA. | ||||
| 48 | Klinkhardt U, Kirchmaier CM, Westrup D, Graff J, Mahnel R, Breddin HK, Harder S "Ex vivo-in vitro interaction between aspirin, clopidogrel, and the glycoprotein IIb/IIIa inhibitors abciximab and SR121566A." Clin Pharmacol Ther 67 (2000): 305-13. [PMID: 10741635] | ||||
| 49 | Product Information. Xtandi (enzalutamide). Astellas Pharma US, Inc, Deerfield, IL. | ||||
| 50 | Product Information. Flolan (epoprostenol). Glaxo Wellcome, Research Triangle Park, NC. | ||||
| 51 | Abebe W "Herbal medication: potential for adverse interactions with analgesic drugs." J Clin Pharm Ther 27 (2002): 391-401. [PMID: 12472978] | ||||
| 52 | Bergmann TK, Filppula AM, Launiainen T, Nielsen F, Backman J, Brosen K "Neurotoxicity and low paclitaxel clearance associated with concomitant clopidogrel therapy in a 60 year old Caucasian woman with ovarian carcinoma." Br J Clin Pharmacol (2015):. [PMID: 26446447] | ||||
| 53 | Product Information. Bevyxxa (betrixaban). Portola Pharmaceuticals, South San Francisco, CA. | ||||
| 54 | Ohyama K, Nakajima M, Suzuki M, Shimada N, Yamazaki H, Yokoi T "Inhibitory effects of amiodarone and its N-deethylated metabolite on human cytochrome P450 activities: Prediction of in vivo drug interactions." Br J Clin Pharmacol 49 (2000): 244-53. [PMID: 10718780] | ||||
