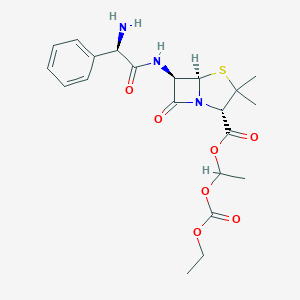
| Synonyms |
Bacampicilina; Bacampicilline; Bacampicillinum; Penglobe; Bacampicillin hydrochloride; Bacampicilina [INN-Spanish]; Bacampicillin (INN); Bacampicillin [INN:BAN]; Bacampicilline [INN-French]; Bacampicillinum [INN-Latin]; Penglobe (TN); Spectrobid (TN); (2S,5R,6R)-6((R)-(2-Amino-2-phenylacetamido))-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid ester with ethyl 1-hydroxyethylcarbonate; 1'-Ethoxycarbonyloxyethyl-(6-D-alpha-aminophenylacetamido)penicillanate; 1-Ethoxycarbonyloxyethyl (2S,5R,6R)-6((R)-(2-amino-2-phenylacetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptan-2-carboxylat; 1-[(ethoxycarbonyl)oxy]ethyl (2S,5R,6R)-6-{[(2R)-2-amino-2-phenylacetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate; 1-[(ethoxycarbonyl)oxy]ethyl 6beta-[(2R)-2-amino-2-phenylacetamido]-2,2-dimethylpenam-3alpha-carboxylate; 1-ethoxycarbonyloxyethyl (2S,5R)-6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate; 1-ethoxycarbonyloxyethyl (2S,5R,6R)-6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate; 1-ethoxycarbonyloxyethyl (2S,5R,6R)-6-[[(2S)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate; 6-((R)-2-Amino-2-phenylacetamido)penicillansaeure-(1-(ethoxycarbonyloxy)ethylester
|