Details of the Drug
General Information of Drug (ID: DM1237M)
| Drug Name |
Alfacalcidol
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alfacalcidol [INN:BAN:JAN]; Alfacalcidolum; Alfacalcidolum [INN-Latin]; Alfarol; Alpha D 3; Alphacalcidol; Alsiodol; Bondiol; EinsAlpha; One-Alpha; Oxydevit; Sinovul; URQ2517572; Un-Alpha; Vitamin D3, 1alpha-Hydroxy-; alfacalcidol; alpha-Calcidol; (5Z,7E)-9,10-Seco-5,7,10(19)-cholestatrien-1alpha,3beta-diol; 1-Hydroxycholecalciferol; 1-Hydroxyvitamin D3; 1alpha-Hydroxy-vitamin D3; 1alpha-Hydroxycholecalciferol; 1alpha-Hydroxyvitamin D3; 1alpha-OH-D3; 41294-56-8; CCRIS 3341; CHEBI:31186; UNII-URQ2517572
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||
| Structure |
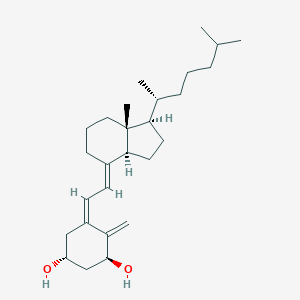 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 400.6 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 6.8 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
