Details of the Drug
General Information of Drug (ID: DM1XT4N)
| Drug Name |
Tafenoquine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms | 106635-80-7; WR-238605; WR 238605; Etaquine; WR238605; Tafenoquine [INN:BAN | ||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Plasmodium
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
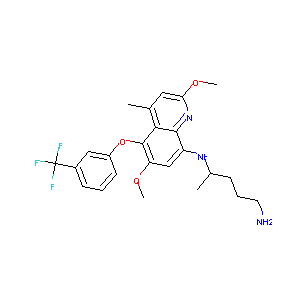 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 463.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.4 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Tafenoquine: First Global Approval.Drugs. 2018 Sep;78(14):1517-1523. | ||||
|---|---|---|---|---|---|
| 2 | Deshmukh R, Sharma L, Tekade M, Kesharwani P, Trivedi P, Tekade RK: Force degradation behavior of glucocorticoid deflazacort by UPLC: isolation, identification and characterization of degradant by FTIR, NMR and mass analysis. J Biomed Res. 2016 Mar;30(2):149-161. doi: 10.7555/JBR.30.20150074. Epub 2016 Feb 20. | ||||
| 3 | Oral lipid-based nanoformulation of tafenoquine enhanced bioavailability and blood stage antimalarial efficacy and led to a reduction in human red blood cell loss in mice. Int J Nanomedicine. 2015 Feb 20;10:1493-503. doi: 10.2147/IJN.S76317. eCollection 2015. | ||||
| 4 | Tafenoquine and its potential in the treatment and relapse prevention of Plasmodium vivax malaria: the evidence to date. Drug Des Devel Ther. 2016 Jul 26;10:2387-99. | ||||
