Details of the Drug
General Information of Drug (ID: DM25CGV)
| Drug Name |
Selenium
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Selenium; Selenium alloy; Selenium and compounds; Selenium anhydride; Selenium base; Selenium dihydride; Selenium dust; Selenium elemental; Selenium homopolymer; Selenium hydride; Selenium metallicum; Selenium, colloidal; Selenium, elemental; Selenium, metallic; Vandex; selenio; 7782-49-2; CCRIS 4250; Caswell No. 732; EINECS 231-957-4; Colloidal selenium; Electronic E-2; Elemental selenium; Gray selenium; Hydrogen selenide (H2Se); SELENIUM ATOM; SELENIUM COMPOUNDS; SELENIUM POWDER; Selen [Polish]; HSDB 4493; Se; Selen; UNII-H6241UJ22B
|
||||||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||||||
| Structure |
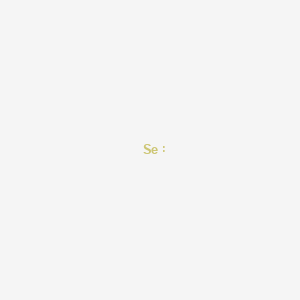 |
||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 78.97 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||||||||||
| Rotatable Bond Count | 0 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 0 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 0 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
