Details of the Drug
General Information of Drug (ID: DM2WE5K)
| Drug Name |
Ceruletide
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Caerulein; Cerulein; Ceruletida; Ceruletidum; Ceruletida [INN-Spanish]; Ceruletidum [INN-Latin]; Ceruletide (USAN/INN); Ceruletide [USAN:INN:BAN]; 5-Oxo-L-prolyl-L-glutaminyl-L-alpha-aspartyl-O-sulfo-L-tyrosyl-L-threonylglycyl-L-tryptophyl-L-methionyl-L-alpha-aspartyl-L-phenylalaninamide; 5-Oxo-L-prolyl-L-glutaminyl-L-aspartyl-L-tyrosyl-L-threonylglycyl-L-tryptophyl-L-methionyl-L-aspartylphenyl-L-alaninamide 4-(hydrogen sulfate) (ester); 5-oxo-L-prolyl-L-glutaminyl-L-a-aspartyl-O-sulfo-L-tyrosyl-L-threonylglycyl-L-tryptophyl-L-methionyl-L-a-aspartyl-L-phenylalaninamide
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Diagnostic Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
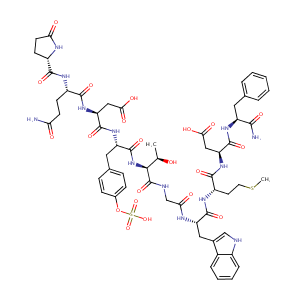 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 5 | Molecular Weight (mw) | 1352.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -3 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 38 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 17 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 22 | ||||||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Caerulein stimulated gastric and pancreatic secretion | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5A4Y | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
