Details of the Drug
General Information of Drug (ID: DM62IPN)
| Drug Name |
Tenofovir disoproxil
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Tenofovir (Disoproxil); Tenofovir disoproxil; 201341-05-1; 9-((R)-2-((Bis(((isopropoxycarbonyl)oxy)methoxy)phosphinyl)methoxy)propyl)adenine; Bis(POC)PMPA; PMPA prodrug; CHEBI:63717; F4YU4LON7I; UNII-F4YU4LON7I; [[(1R)-2-(6-aminopurin-9-yl)-1-methyl-ethoxy]methyl-(isopropoxycarbonyloxymethoxy)phosphoryl]oxymethyl isopropyl carbonate; tenofovir bis(isopropyloxycarbonyloxymethyl) ester
|
|||||
| Affected Organisms |
Human Immunodeficiency Virus
|
|||||
| ATC Code | ||||||
| Structure |
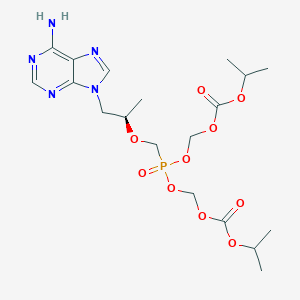 |
|||||
| 3D MOL is unavailable | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 519.4 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 1.6 | |||||
| Rotatable Bond Count (rotbonds) | 17 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 14 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||
Molecular Interaction Atlas of This Drug
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
