Details of the Drug
General Information of Drug (ID: DM6GTMD)
| Drug Name |
ADX-48621
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dipraglurant; 872363-17-2; UNII-CV8JZR21A1; ADX48621; CV8JZR21A1; CHEMBL2346738; 6-fluoro-2-[4-(2-pyridinyl)-3-butyn-1-yl]-Imidazo[1,2-a]pyridine; Dipraglurant [INN]; ADX-48621; SCHEMBL103033; GTPL6452; C16H12FN3; DTXSID90236230; BCP23706; BDBM50431705; ZINC72266314; 3590AH; 6-Fluoro-2-(4-(pyridin-2-yl)but-3-yn-1-yl)imidazo(1,2-a)pyridine; AKOS030526956; SB16993; DB12733; CS-0690; NCGC00351745-02; HY-14859; DB-076892; FT-0705373; J3.560.334D; W-5761; ADX 48621;ADX48621;ADX-48621
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
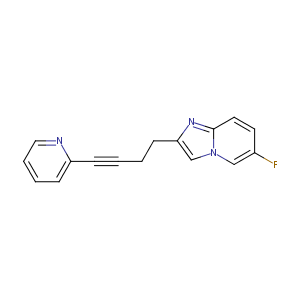 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 265.28 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.5 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Parkinson disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A00.0 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
