Details of the Drug
General Information of Drug (ID: DM7TZL0)
| Drug Name |
INCB054329
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1628607-64-6; (S)-6-(3,5-dimethylisoxazol-4-yl)-3-(pyridin-2-yl)-3,4-dihydro-5-oxa-1,2a-diazaacenaphthylen-2(1H)-one; Q50825078; SCHEMBL16038298; INCB54329; US9624241, Example 14; BDBM318469; INCB-54329; EX-A3126; INCB-054329; NSC816024; s8753; NSC-816024; HY-112504; CS-0046160; INCB054329 pound INCB-054329,INCB-54329 pound(c); CC1=NOC(C)=C1C1=CC=C2C3=C1OC[C@H](C=1N=CC=CC=1)N3C(=O)N2; (4S)-7-(3,5-dimethylisoxazol-4-yl)-4-pyridin-2-yl-4,5-dihydroimidazo[1,5,4-de][1,4]benzoxazin-2(1H)-one
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
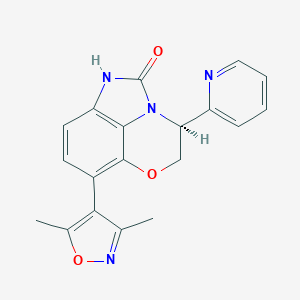 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 348.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
