Details of the Drug
General Information of Drug (ID: DMBG0N4)
| Drug Name |
Cibenzoline
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Cibenzolina; Cibenzolina [INN-Spanish]; Cibenzoline; Cibenzoline (INN); Cibenzoline [INN]; Cibenzolinum; Cibenzolinum [INN-Latin]; Cifenline; Cifenline (USAN); Cifenline [USAN]; Ro 22-7796; Ro 227796; UP 33901; (+-)-2-(2,2-Diphenylcyclopropyl)-2-imidazoline; 1H-Imidazole, 2-(2,2-diphenylcyclopropyl)-4,5-dihydro-, (+-)-; 2-(2,2-Diphenyl-cyclopropyl)-4,5-dihydro-1H-imidazole; 2-(2,2-diphenylcyclopropyl)-2-imidazoline; 2-(2,2-diphenylcyclopropyl)-4,5-dihydro-1H-imidazole; 53267-01-9; C18H18N2; CHEMBL87045; EINECS 258-453-7
|
|||||
| ATC Code | ||||||
| Structure |
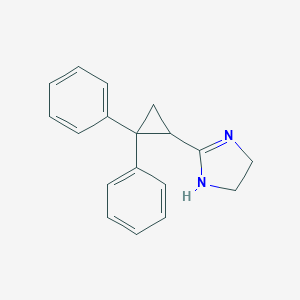 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 262.3 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 2.7 | |||||
| Rotatable Bond Count (rotbonds) | 3 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
