Details of the Drug
General Information of Drug (ID: DMBIJZ6)
| Drug Name |
Tocopherol
|
|||||
|---|---|---|---|---|---|---|
| Synonyms | Methyltocols; tocoferol; tocoferoles; tocophrol; Tocopherols | |||||
| Affected Organisms |
Humans and other mammals
|
|||||
| Structure |
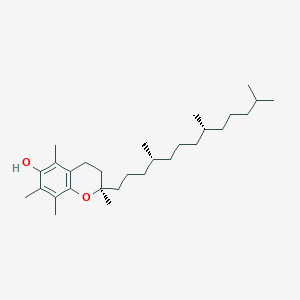 |
|||||
| 3D MOL | 2D MOL | |||||
| ADMET Property |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
