Details of the Drug
General Information of Drug (ID: DMBJMZC)
| Drug Name |
GSK239512
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
720691-69-0; GSK-239512; 1-(6-((3-CYCLOBUTYL-2,3,4,5-TETRAHYDRO-1H-BENZO[D]AZEPIN-7-YL)OXY)PYRIDIN-3-YL)PYRROLIDIN-2-ONE; GSK239512; UNII-4I7U5C459M; CHEMBL3092650; 4I7U5C459M; YFRBKEVUUCQYOW-UHFFFAOYSA-N; SCHEMBL167578; MolPort-035-776-189; ZINC3961802; BDBM50444496; AKOS025291102; SB16754; KS-0000063Q; AS-42474; AK171368; 2-Pyrrolidinone, 1-(6-((3-cyclobutyl-2,3,4,5-tetrahydro-1H-3-benzazepin-7-yl)oxy)-3-pyridinyl)-; J3.497.402K
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
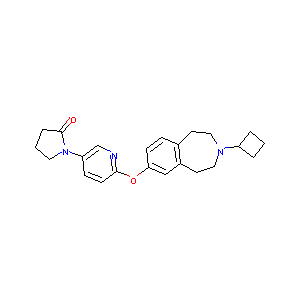 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 377.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.4 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Alzheimer disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 8A20 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
