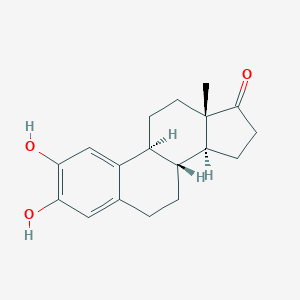
| Synonyms |
Catecholestrone; LMST02010032; SCHEMBL222517; UQS3A06ILY; ZINC4096681; 2,3-Dihydroxyestra-1,3,5(10)-trien-17-one; 2,3-dihydroxy-estra-1,3,5(10)-trien-17-one; 2-HYDROXYESTRONE; 2-Hydroxy Estrone; 2-Hydroxyestrone; 2-OHE1; 362-06-1; 362H061; AC1L99N9; AKOS030254485; C-44096; C05298; CCRIS 9276; CHEBI:1156; CHEMBL1627343; CTK4H6079; DTXSID80904315; Estra-1,3,5(1)-trien-17-one, 2,3-dihydroxy-; Estra-1,3,5(10)-trien-17-one, 2,3-dihydroxy-; Estra-1,3,5(10)-trien-17-one,2,3-dihydroxy-; UNII-UQS3A06ILY
|
| Chemical Identifiers |
- Formula
- C18H22O3
- IUPAC Name
(8R,9S,13S,14S)-2,3-dihydroxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-one - Canonical SMILES
-
CC12CCC3C(C1CCC2=O)CCC4=CC(=C(C=C34)O)O
- InChI
-
SWINWPBPEKHUOD-JPVZDGGYSA-N
- InChIKey
-
1S/C18H22O3/c1-18-7-6-11-12(14(18)4-5-17(18)21)3-2-10-8-15(19)16(20)9-13(10)11/h8-9,11-12,14,19-20H,2-7H2,1H3/t11-,12+,14-,18-/m0/s1
|