Details of the Drug
General Information of Drug (ID: DMBXWU8)
| Drug Name |
Aprindine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Aprindin; Aprindina; Aprindinum; Aprinidine; Fibocil; AC 1802; Aprindina [INN-Spanish]; Aprindinum [INN-Latin]; Aprindine (USAN/INN); Aprindine [USAN:BAN:INN]; N-(3-(Diethylamino)propyl)-N-phenyl-2-indanamine; N,N-Diethyl-N'-2-indanyl-N'-phenyl-1,3-propanediamine; N,N-Diethyl-N'-2-indanyl-N'-phenyl-1,3-propanediaminemonohydrochloride; N'-(2,3-dihydro-1H-inden-2-yl)-N,N-diethyl-N'-phenylpropane-1,3-diamine; N-(2,3-Dihydro-1H-inden-2-yl)-N',N'-diethyl-N-phenyl-1,3-propanediamine; N-(2,3-dihydro-1H-inden-2-yl)-N',N'-diethyl-N-phenylpropane-1,3-diamine
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antiarrhythmic Agents
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
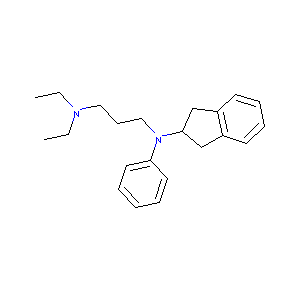 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 322.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.2 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 8 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Cardiac arrhythmias | |||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BC9Z | |||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||||||||||||||
References
