Details of the Drug
General Information of Drug (ID: DMC50VB)
| Drug Name |
Orforglipron
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Orforglipron; LY3502970; 2212020-52-3; CHEMBL4446782; LY-3502970; 3-[(1S,2S)-1-(5-[(4S)-2,2-dimethyloxan-4-yl]-2-{(4S)-2-(4-fluoro-3,5-dimethylphenyl)-3-[3-(4-fluoro-1-methyl-1H-indazol-5-yl)-2-oxo-2,3-dihydro-1H-imidazol-1-yl]-4-methyl-2,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridine-5-carbonyl}-1H-indol-1-yl)-2-methylcyclopropyl]-1,2,4-oxadiazol-5(4H)-one; 3-[(1S,2S)-1-[5-[(4S)-2,2-dimethyloxan-4-yl]-2-[(4S)-2-(4-fluoro-3,5-dimethylphenyl)-3-[3-(4-fluoro-1-methylindazol-5-yl)-2-oxoimidazol-1-yl]-4-methyl-6,7-dihydro-4H-pyrazolo[4,3-c]pyridine-5-carbonyl]indol-1-yl]-2-methylcyclopropyl]-4H-1,2,4-oxadiazol-5-one; V6G; orforglipron [INN]; ORFORGLIPRON [USAN]; OWL833; SCHEMBL21175277; GTPL12175; 7ZW40D021M; EX-A7751; BDBM50514045; AKOS040733262; GLP-1 receptor agonist 1;Orforglipron; MS-31635; HY-112185; CS-0043632; 1,2,4-Oxadiazol-5(2H)-one, 3-[(1S,2S)-1-[2-[[(4S)-2-(4-fluoro-3,5-dimethylphenyl)-3-[3-(4-fluoro-1-methyl-1H-indazol-5-yl)-2,3-dihydro-2-oxo-1H-imidazol-1-yl]-2,4,6,7-tetrahydro-4-methyl-5H-pyrazolo[4,3-c]pyridin-5-yl]carbonyl]-5-[(4S)-tetrahydro-2,2-dimethyl-2H-pyran-4-yl]-1H-indol-1-yl]-2-methylcyclopropyl]-; 3-[(1S,2S)-1-({2-(4-fluoro-3,5-dimethylphenyl)-3-({3-[3-(4-fluoro-1-methyl-1H-indazol-5-yl)-2-oxo-2,3-dihydro-1H-imidazol-1-yl]-4-methyl-2,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridin-5-yl}carbonyl)-5-[(4S)-2,2-dimethyloxan-4-yl]-1H-indol-1-yl}-2-methylcyclopropyl]-5-oxo-1,2,4-oxadiazol-4(5H)-ide
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecule
|
||||||||||||||||||||||||||
| Structure |
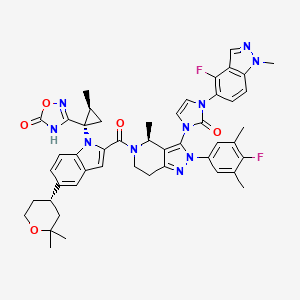 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Obesity | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 5B81 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
