Details of the Drug
General Information of Drug (ID: DMCLHO0)
| Drug Name |
Bethanechol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Amidopropyldimethylbetaine; Bethanecol; Urabeth; Myotonine chloride; Duvoid (TN); Myotonachol (TN); Urecholine (TN); Carbamoyl-beta-methylcholine; Carbamyl-beta-methylcholine; Ammonium, (2-hydroxypropyl)trimethyl-, carbamate (ester); (2-Hydroxypropyl)trimethylammonium carbamate; 1-Propanaminium, 2-((aminocarbonyl)oxy)-N,N,N-trimethyl-(9CI); 2-((Aminocarbonyl)oxy)-N,N,N-trimethyl-1-propanaminium; 2-(carbamoyloxy)-N,N,N-trimethylpropan-1-aminium; 2-carbamoyloxypropyl(trimethyl)azanium; 2-carbamoyloxypropyl-trimethylazanium
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Parasympathomimetics
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
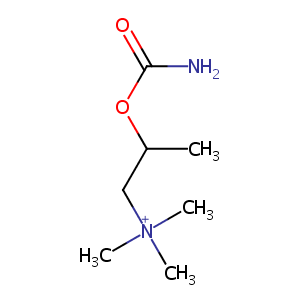 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 161.22 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Bethanechol (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 297). | ||||
|---|---|---|---|---|---|
| 2 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 3 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 4 | Loss of Ca-mediated ion transport during colitis correlates with reduced ion transport responses to a Ca-activated K channel opener. Br J Pharmacol. 2009 Apr;156(7):1085-97. | ||||
| 5 | Benjamin KW "Toxicity of ocular medications." Int Ophthalmol Clin 19 (1979): 199-255. [PMID: 376469] | ||||
| 6 | Product Information. Suprep Bowel Prep Kit (magnesium/potassium/sodium sulfates). Braintree Laboratories, Braintree, MA. | ||||
| 7 | Product Information. Aricept (donepezil). Pfizer US Pharmaceuticals, New York, NY. | ||||
| 8 | Product Information. Wellbutrin XL (buPROPion). GlaxoSmithKline, Philadelphia, PA. | ||||
