Details of the Drug
General Information of Drug (ID: DMDKSZ0)
| Drug Name |
2-oxo-N-m-tolyl-2H-chromene-3-carboxamide
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1846-99-7; 2-oxo-N-(m-tolyl)-2H-chromene-3-carboxamide; 2-Oxo-2H-chromene-3-carboxylic acid m-tolylamide; N-(3-Methylphenyl)-2-oxo-2H-chromene-3-carboxamide; AC1LCCPQ; BAS 00837918; AC1Q2NKJ; 3-carboxamido coumarin, 8; TimTec1_006709; Oprea1_808053; Oprea1_256730; CBDivE_000076; MLS001209245; CHEMBL445184; SCHEMBL6228552; BDBM29158; 2h-1-benzopyran-3-carboxamide,n-(3-methylphenyl)-2-oxo-; MolPort-000-375-220; XUNAVIWFCXQWCJ-UHFFFAOYSA-N; HMS2825F09; HMS1553A21; ZINC130233; N-(m-Tolyl)-3-coumarincarboxamide; STK408207
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
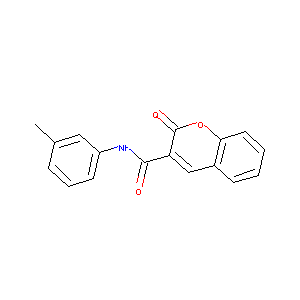 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 279.29 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
