Details of the Drug
General Information of Drug (ID: DMEH3O7)
| Drug Name |
Rifamycin
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Rifamycin SV; Rifamycin SV MMX; Rifamycin sodium; CB-01-11; Rifamycin (oral controlled-release, gastrointestinal-specific); Rifamycin (oral controlled-release, gastrointestinal-specific), Cosmo/Dr Falk/Santarus
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Escherichia coli
|
||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
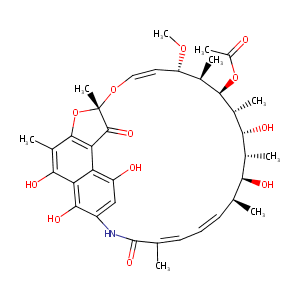 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 | Molecular Weight (mw) | 697.8 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 6 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 12 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Rifamycin action on RNA polymerase in antibiotic-tolerant Mycobacterium tuberculosis results in differentially detectable populations.Proc Natl Acad Sci U S A. 2017 Jun 13;114(24):E4832-E4840. | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4570). | ||||
| 3 | Systemic absorption of rifamycin SV MMX administered as modified-release tablets in healthy volunteers. Antimicrob Agents Chemother. 2011 May;55(5):2122-8. doi: 10.1128/AAC.01504-10. Epub 2011 Mar 14. | ||||
| 4 | NCBI report | ||||
| 5 | Multiple intra-articular treatment of rheumatoid arthritis: a randomized prospective study comparing rifamycin SV with pefloxacin. J Int Med Res. 1992 Feb;20(1):27-39. doi: 10.1177/030006059202000104. | ||||
| 6 | The influence of picolines on glutathione transferase activity and subunit composition in human liver derived Hep G2 cells. Biochem Pharmacol. 1994 Nov 16;48(10):1976-8. | ||||
| 7 | Differential interaction of 3-hydroxy-3-methylglutaryl-coa reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab Dispos. 2005 Apr;33(4):537-46. | ||||
| 8 | Neonicotinoid pesticides poorly interact with human drug transporters. J Biochem Mol Toxicol. 2019 Oct;33(10):e22379. doi: 10.1002/jbt.22379. Epub 2019 Jul 31. | ||||
