Details of the Drug
General Information of Drug (ID: DMELOIQ)
| Drug Name |
ALPHA-NAPHTHOFLAVONE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
7,8-Benzoflavone; alpha-Naphthoflavone; 604-59-1; 2-Phenyl-4H-benzo[h]chromen-4-one; alpha-Naphthylflavone; 2-phenylbenzo[h]chromen-4-one; Benzo(h)flavone; 7,8-BF; 4H-Naphtho[1,2-b]pyran-4-one, 2-phenyl-; .alpha.-Naphthoflavone; 2-Phenyl-benzo[h]chromen-4-one; 2-Phenyl-4H-naphtho(1,2-b)pyran-4-one; CCRIS 3607; 2-Phenylbenzo(h)chromen-4-one; EINECS 210-071-1; UNII-FML65D8PY5; NSC 407011; BRN 0210862; FML65D8PY5; benzo[h]flavone; MLS003171601; CHEMBL283196; CHEBI:76995; VFMMPHCGEFXGIP-UHFFFAOYSA-N; 4H-NAPHTHO(1,2-b)PYRAN-4-ONE, 2-PH
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
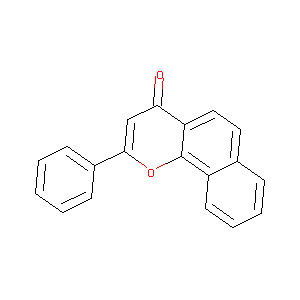 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 272.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 4.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
