Details of the Drug
General Information of Drug (ID: DMFJH5Q)
| Drug Name |
Mephentermine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Mefenterdrin; Mefentermin; Mefentermina; Mephenterdrine; Mephenterdrinum; Mephenterminum; Mephetedrine; Mephine; Vialin; Wyamine; Wyfentermina; Mefentermina [INN-Spanish]; Mephentermine (INN); Mephentermine [INN:BAN]; Mephenterminum [INN-Latin]; N-Methylphentermine; WY-585; Mephentermine Sulfate (2:1); N,alpha,alpha-Trimethylbenzeneethanamine; N,alpha,alpha-Trimethylphenethylamine; Omega-Phenyl-tert-butyl-methylamine; N-Methyl-omega-phenyl-t-butylamine; N-Methyl-omega-phenyl-tert-butylamine; N,2-dimethyl-1-phenylpropan-2-amine; 2-Methyl-2-methylamino-1-phenylpropane; 2-Methylamino-2-methyl-1-phenylpropane
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Vasoconstrictor Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
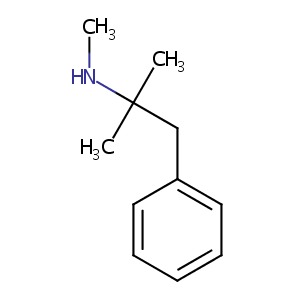 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 163.26 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | High blood pressure | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | BA00 | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Mephentermine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
