Details of the Drug
General Information of Drug (ID: DMJAPE7)
| Drug Name |
Methylene blue
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
methylene blue; 61-73-4; Methylthioninium chloride; Swiss Blue; Chromosmon; Methylene Blue N; Methylenium ceruleum; Urolene blue; Methylene Blue chloride; Solvent blue 8; Bleu de methylene; Methylene Blue G; Methylene Blue A; External Blue 1; Methylene Blue D; Methylene Blue B; Methylene Blue anhydrous; CI Basic Blue 9; Methylene Blue ZF; Methylene Blue SP; Methylene Blue NZ; Methylene Blue BX; Methylene Blue BD; Methylene Blue SG; Tetramethylene Blue; Methylene Blue ZX; Methylene Blue FZ; Methylene Blue BP; Calcozine; 3,7-Bis-dimethylamino-phenothiazin-5-ylium
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Structure |
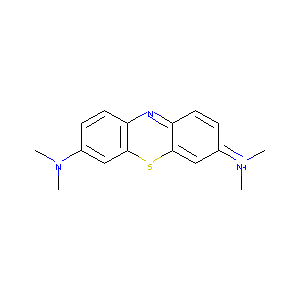 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 319.9 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||||||
| Rotatable Bond Count | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 4 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Methylene blue (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||
References
