Details of the Drug
General Information of Drug (ID: DMFSWGH)
| Drug Name |
Econazole
|
||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Ecostatin; Palavale; Pevaryl; Ecostatin Vaginal Ovules; Ecostatin cream; Spectazole cream; SQ 13050; Ecostatin (TN); Gyno-Pevaryl 150; Gyno-pevaryl; Pevaryl (TN); Spectazole (TN); Econazole (USAN/INN); 1-[2,4-Dichloro-.beta.-[(p-chlorobenzyl)oxy]phenethyl]imidazole; 1-[2-[(4-Chlorophenyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-1H-imidazole; 1-[2-[(4-chlorophenyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole; 1-{2-(4-chlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole; 1-{2-[(4-chlorobenzyl)oxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole; 1-{2-[(4-chlorophenyl)methoxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antifungal Agents
|
||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Yeast and other fungi
|
||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
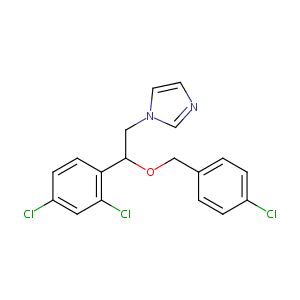 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 381.7 | |||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.3 | ||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 6 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||||||||||||||
| ADMET Property | |||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
References
