| Drug Name |
Ebastine
|
| Synonyms |
ebastine; Kestine; Ebastel; Ebastin; Evastel; Kestin; Bactil; Ebastinum [Latin]; LAS W-090; Ebastina [Spanish]; Estivan; UNII-TQD7Q784P1; RP 64305; 1-(4-tert-butylphenyl)-4-[4-(diphenylmethoxy)piperidin-1-yl]butan-1-one; TQD7Q784P1; CHEMBL305660; 4-Diphenylmethoxy-1-(3-(4-tert-butylbenzoyl)propyl)piperidine; C32H39NO2; MFCD00865661; 1-[4-(1,1-Dimethylethyl)phenyl]-4-[4-(diphenylmethoxy)-1-piperidinyl]-1-butanone; NCGC00164603-01; RP-64305; 4'-tert-Butyl-4-(4-(diphenylmethoxy)piperidino)butyrophenone
|
| ATC Code |
- R06AX22: Ebastine
- R06AX: Other antihistamines for systemic use
- R06A: ANTIHISTAMINES FOR SYSTEMIC USE
- R06: ANTIHISTAMINES FOR SYSTEMIC USE
- R: RESPIRATORY SYSTEM
|
| Structure |
|
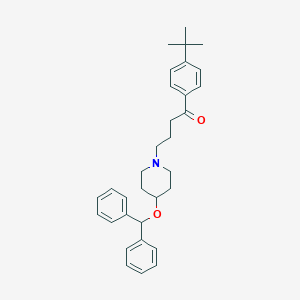 |
|
3D MOL
|
2D MOL
|
#Ro5 Violations (Lipinski):
0 |
Molecular Weight |
469.7 |
| Logarithm of the Partition Coefficient |
Not Available |
| Rotatable Bond Count |
10 |
| Hydrogen Bond Donor Count |
0 |
| Hydrogen Bond Acceptor Count |
3 |
| Chemical Identifiers |
- Formula
- C32H39NO2
- IUPAC Name
4-(4-benzhydryloxypiperidin-1-yl)-1-(4-tert-butylphenyl)butan-1-one - Canonical SMILES
-
CC(C)(C)C1=CC=C(C=C1)C(=O)CCCN2CCC(CC2)OC(C3=CC=CC=C3)C4=CC=CC=C4
- InChI
-
InChI=1S/C32H39NO2/c1-32(2,3)28-18-16-25(17-19-28)30(34)15-10-22-33-23-20-29(21-24-33)35-31(26-11-6-4-7-12-26)27-13-8-5-9-14-27/h4-9,11-14,16-19,29,31H,10,15,20-24H2,1-3H3
- InChIKey
-
MJJALKDDGIKVBE-UHFFFAOYSA-N
|
| Cross-matching ID |
- PubChem CID
- 3191
- ChEBI ID
-
- CAS Number
-
- UNII
-
- DrugBank ID
-
- VARIDT ID
- DR01688
|
|
|
|
|
|
|
|




