Details of the Drug
General Information of Drug (ID: DMH2CKX)
| Drug Name |
METHOCTRAMINE
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Methoctramine; CHEBI:73339; UNII-NVJ76B897D; CHEMBL27673; NVJ76B897D; n,n'-bis{6-[(2-methoxybenzyl)amino]hexyl}octane-1,8-diamine; N1,N8-bis(6-(2-methoxybenzylamino)hexyl)octane-1,8-diamine; N,N'-bis[6-[(2-Methoxyphenyl)methylamino]hexyl]octane-1,8-diamine; N,N'-Bis(6-((2-methoxyphenyl)methylamino)hexyl)octane-1,8-diamine; N,N'-Bis[6-[[(2-methoxyphenyl)methyl]amino]hexyl]-1,8-octanediamine; N,N'-Bis(6-(((2-methoxyphenyl)methyl)amino)hexyl)-1,8-octanediamine; Methoctramine free base; NCGC00015626-01; Lopac-M-105
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
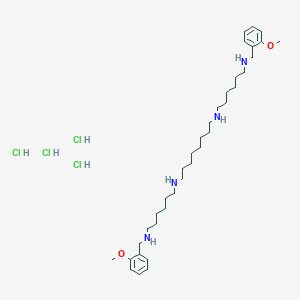 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 3 |
Molecular Weight | 728.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 29 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 8 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 6 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
