Details of the Drug
General Information of Drug (ID: DMHXTAQ)
| Drug Name |
Pazufloxacin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
127045-41-4; T-3761; UNII-4CZ1R38NDI; 4CZ1R38NDI; NCGC00167534-01; DSSTox_CID_26697; DSSTox_RID_81831; DSSTox_GSID_46697; Pazufloxacin [INN]; SMR000466380; CCRIS 7312; CAS-127045-41-4; T 3761; 127046-18-8; PZFX; Pazufloxacin (JAN/INN); SCHEMBL34460; 7H-Pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid, 10-(1-aminocyclopropyl)-9-fluoro-2,3-dihydro-3-methyl-7-oxo-, (S)-; MLS000759513; MLS001424116; CHEMBL240163; DTXSID5046697; CHEBI:94700; HY-B0724B; HMS2051B05; HMS2090H07; BCP12954; LSM-5745; RKL10069; ZINC3779726; Tox21_112531; BDBM50248017; MFCD00865012; AKOS015900451; Tox21_112531_1; AC-3506; CCG-100919; DB11774; GM-1171; KS-5008; NC00169; NCGC00167534-02; (-)-(3S)-10-(1-Aminocyclopropyl)-9-fluoro-2,3-dihydro-3-methyl-7-oxo-7H-pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid; 7H-Pyrido(1,2,3-de)-1,4-benzoxazine-6-carboxylic acid, 2,3-dihydro-10-(1-aminocyclopropyl)-9-fluoro-3-methyl-7-oxo-, (S)-; AB0012715; D01153; AB00639918-07; AB00639918-09; AB00639918_10; Pazufloxacin, VETRANAL(TM), analytical standard; SR-01000759348; J-005457; Q3898423; SR-01000759348-4; (S)-10-(1-aminocyclopropyl)-9-fluoro-3-methyl-7-oxo-3,7-dihydro-2H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid; (S)-10-(1-aminocyclopropyl)-9-fluoro-3-methyl-7-oxo2,3-dihydro-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxylic acid; 7H-Pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid,10-(1-aminocyclopropyl)-9-fluoro-2,3-dihydro-3-methyl-7-oxo-, (3S)-; 7H-Pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylicacid,10-(1-aminocyclopropyl)-9-fluoro-2,3-dihydro-3-methyl-7-oxo-
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
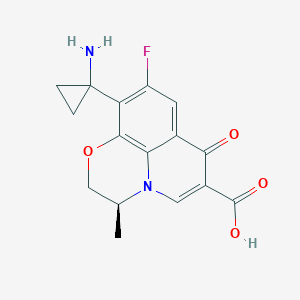 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 318.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.8 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
