Details of the Drug
General Information of Drug (ID: DMIO4V5)
| Drug Name |
VRX496
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Nelfinavir; nelfinavir; 159989-64-7; Viracept; Nelfinavir [INN:BAN]; AG1343; UNII-HO3OGH5D7I; AG-1343; C32H45N3O4S; NELFINAVIR MESYLATE AG1343; Viracept (TN); HO3OGH5D7I; CHEBI:7496; AG 1343; NLF; 1UN; 2-[2-HYDROXY-3-(3-HYDROXY-2-METHYL-BENZOYLAMINO)-4-PHENYL SULFANYL-BUTYL]-DECAHYDRO-ISOQUINOLINE-3-CARBOXYLIC ACID TERT-BUTYLAMIDE; (3S-(2(2S*,3S*),3alpha,4abeta,8abeta))-N-(1,1-Dimethylethyl)decahydro-2-(2-hydroxy-3-((3-hydroxy-2-methylbenzoyl)amino)-4-(phenylthio)butyl)-3-isoquinolinecarboxamide; NFV; NFV; Nelfinavir Monomethane Sulfonate; AG1346; Nelfinavir (INN); Nelfinavir [BAN:INN]; AG1343 (*Mesylate salt*); Viracept (TM)(*Mesylate salt*); Met-SDF-1beta & Nelfinavir; Met-Stromal Cell-derived Factor-1beta (Human) & Nelfinavir; (3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-phenylsulfanylbutyl]-3,4,4a,5,6,7,8,8a-octahydro-1H-isoquinoline-3-carboxamide
|
|||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antisense
|
|||||||||||||||||||||||||||||||||||
| Drug Type |
Antisense drug
|
|||||||||||||||||||||||||||||||||||
| Structure |
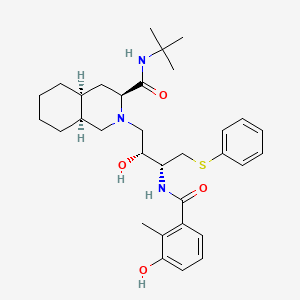 |
|||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 2 | Molecular Weight (mw) | 567.8 | ||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 5.7 | |||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 10 | |||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | |||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | |||||||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
|||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
