Details of the Drug
General Information of Drug (ID: DMJ18YL)
| Drug Name |
Secnidazole
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
3366-95-8; Secnidazol; Secnidazolum; Flagentyl; Secnidazolum [INN-Latin]; Solosec; RP 14539; Secnidazol [INN-Spanish]; PM 185184;Secnidazole (Flagentyl); SYM-1219; EINECS 222-134-0; seknidazol; MLS000559043; PM-185184; NCGC00095158-01; SMR000149359; RP-14539
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Bacteroides fragilisTrichomonas vaginalis, Giardia duodenalis, and Entamoeba histolyticaBacteria and protozoa
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
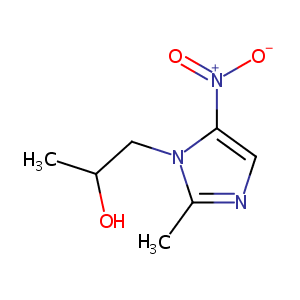 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 185.18 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.2 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | ||||
| 3 | Gillis JC, Wiseman LR: Secnidazole. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis. Drugs. 1996 Apr;51(4):621-38. | ||||
| 4 | Overexpression, isotopic labeling, and spectral characterization of Enterobacter cloacae nitroreductase. Protein Expr Purif. 1998 Jun;13(1):53-60. | ||||
