Details of the Drug
General Information of Drug (ID: DMJFBQ1)
| Drug Name |
9-phenanthrol
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Phenanthren-9-ol; 9-PHENANTHROL; 9-Hydroxyphenanthrene; 9-Phenanthrenol; 484-17-3; UNII-9FYU45OV9H; NSC 50554; CCRIS 1840; EINECS 207-602-4; BRN 2047057; 9FYU45OV9H; CHEBI:28820; DZKIUEHLEXLYKM-UHFFFAOYSA-N; AC1Q7B0Z; AC1L1UR8; DSSTox_RID_82439; DSSTox_CID_27592; ACMC-1AD67; DSSTox_GSID_47592; 4-06-00-04937 (Beilstein Handbook Reference); SCHEMBL508755; 9-Phenanthrol, technical grade; GTPL4114; CHEMBL2407182; DTXSID9047592; CTK1D6170; DZKIUEHLEXLYKM-UHFFFAOYSA-; MolPort-001-787-230; ZINC967824; KS-000018CG; NSC50554; AC1Q7986
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
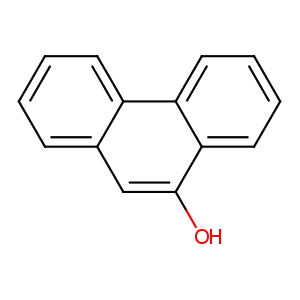 |
||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 194.23 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.6 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
