Details of the Drug
General Information of Drug (ID: DMJFD2I)
| Drug Name |
ABT-492
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Delafloxacin; 189279-58-1; ABT-492; WQ-3034; Abt 492; 1-(6-AMINO-3,5-DIFLUOROPYRIDIN-2-YL)-8-CHLORO-6-FLUORO-7-(3-HYDROXYAZETIDIN-1-YL)-4-OXO-1,4-DIHYDROQUINOLINE-3-CARBOXYLIC ACID; ABT492; RX-3341; UNII-6315412YVF; 6315412YVF; 1-(6-amino-3,5-difluoro-2-pyridyl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxo-quinoline-3-carboxylic acid; 1-(6-amino-3,5-difluoropyridin-2-yl)-8-chloro-6-fluoro-7-(3-hydroxyazetidin-1-yl)-4-oxoquinoline-3-carboxylic acid; Delafloxacin [USAN:INN]; Delafloxacinum; Delafloxacin (USAN)
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Staphylococcus aureusStaphylococcus haemolyticusStaphylococcus lugdunensisStreptococcus pyogenesStreptococcus agalactiaeStreptococcus anginosusEnterococcus faecalisEscherichia coliKlebsiella pneumoniaeEnterobacter cloacaePseudomonas aeruginosaStreptococcus dysgalactiaeEnterobacter aerogenesHaemophilus parainfluenzaeKlebsiella oxytocaProteus mirabilis
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
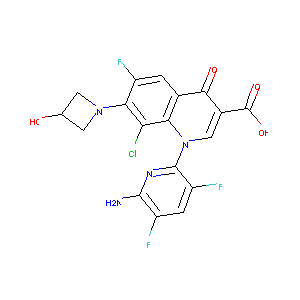 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 440.8 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.7 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 11 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from ABT-492 (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009417) | ||||
|---|---|---|---|---|---|
| 2 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 3 | Contrasting Effects of Acidic pH on the Extracellular and Intracellular Activities of the Anti-Gram-Positive Fluoroquinolones Moxifloxacin and Delafloxacin against Staphylococcus aureus | ||||
| 4 | Delafloxacin: place in therapy and review of microbiologic, clinical and pharmacologic properties. Infect Dis Ther. 2018 Jun;7(2):197-217. | ||||
| 5 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 6 | Product Information. Balversa (erdafitinib). Janssen Products, LP, Horsham, PA. | ||||
| 7 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 8 | Johnson EJ, MacGowan AP, Potter MN, et al "Reduced absorption of oral ciprofloxacin after chemotherapy for haematological malignancy." J Antimicrob Chemother 25 (1990): 837-42. [PMID: 2373666] | ||||
| 9 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 10 | Product Information. Factive (gemifloxacin). GeneSoft Inc, San Francisco, CA. | ||||
| 11 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
