Details of the Drug
General Information of Drug (ID: DMSB068)
| Drug Name |
PF-04449913
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Glasdegib; 1095173-27-5; PF 04449913; UNII-K673DMO5H9; K673DMO5H9; CHEMBL2043437; Glasdegib (PF-04449913); Glasdegib [USAN:INN]; Glasdegib (USAN/INN); PF-04449913;Glasdegib; GTPL8201; Glasdegib(PF-04449913); EX-A858; MolPort-035-789-706; SFNSLLSYNZWZQG-VQIMIIECSA-N; ZINC68251434; PF-913; BDBM50385635; 2640AH; AKOS027324121; CS-2
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
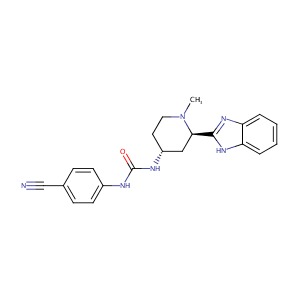 |
||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 374.4 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.4 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Chronic myelomonocytic leukaemia | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A40 | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from PF-04449913 (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | ||||
|---|---|---|---|---|---|
| 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 3 | Minami Y, Minami H, Miyamoto T, Yoshimoto G, Kobayashi Y, Munakata W, Onishi Y, Kobayashi M, Ikuta M, Chan G, Woolfson A, Ono C, Shaik MN, Fujii Y, Zheng X, Naoe T: Phase I study of glasdegib (PF-04449913), an oral smoothened inhibitor, in Japanese patients with select hematologic malignancies. Cancer Sci. 2017 Aug;108(8):1628-1633. doi: 10.1111/cas.13285. Epub 2017 Jun 19. | ||||
| 4 | Imondi AR, Alam AS, Brennan JJ, Hagerman LM: Metabolism of sulpiride in man and rhesus monkeys. Arch Int Pharmacodyn Ther. 1978 Mar;232(1):79-91. | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | ||||
| 7 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | ||||
| 8 | Metabolism, excretion and pharmacokinetics of [14C]glasdegib (PF-04449913) in healthy volunteers following oral administration. Xenobiotica. 2017 Dec;47(12):1064-1076. | ||||
| 9 | Product Information. Fycompa (perampanel). Eisai Inc, Teaneck, NJ. | ||||
| 10 | Product Information. Daurismo (glasdegib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 11 | Product Information. Arcapta Neohaler (indacaterol). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 12 | Cronk GA, Wheatley WB, Fellers GF, Albright H "The relationship of food intake to the absorption of potassium alpha-phenoxyethyl penicillin and potassium phenoxymethyl penicillin from the gastrointestinal tract." Am J Med Sci 240 (1960): 219-25. [PMID: 13812969] | ||||
| 13 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 14 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 15 | Product Information. Retevmo (selpercatinib). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 16 | Product Information. Nurtec ODT (rimegepant). Biohaven Pharmaceuticals, New Haven, CT. | ||||
| 17 | Product Information. Macrilen (macimorelin). Aeterna Zentaris, Charleston, SC. | ||||
| 18 | Product Information. Fareston (toremifene). Schering Laboratories, Kenilworth, NJ. | ||||
| 19 | Product Information. Nubeqa (darolutamide). Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ. | ||||
| 20 | EMA. European Medicines Agency. European Union "EMA - List of medicines under additional monitoring.". | ||||
| 21 | Product Information. Bevyxxa (betrixaban). Portola Pharmaceuticals, South San Francisco, CA. | ||||
