Details of the Drug
General Information of Drug (ID: DMJFZDL)
| Drug Name |
Naphazoline
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Antan; Clearine; Nafazolin; Nafazolina; Nafazoline; Naphazolinum; Naphthizine; Opcon; Nafazolina [DCIT]; Nafazoline [Spanish]; Naphazoline Monohydrochloride; Naphazolinum [Latin]; Enamine_000333; Alpha-Naphthylmethyl imidazoline; Ciba 2020/R; Nafazolin (TN); Naphazoline (INN); Naphazoline [INN:BAN]; Naphazolinum [INN-Latin]; AK-968/41090774; 2-(1-Naphthylmethyl)-2-imidazoline; 2-(1-Naphthylmethyl)-4,5-dihydro-1H-imidazole; 2-(Naphthyl-(1')-methyl)imidazolin; 2-(Naphthyl-(1')-methyl)imidazolin [German]; 2-(alpha-Naphthylmethyl)-imidazoline; 2-(naphthalen-1-ylmethyl)-4,5-dihydro-1H-imidazole; 4,5-Dihydro-2-(1-naphthylmethyl)imidazole
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
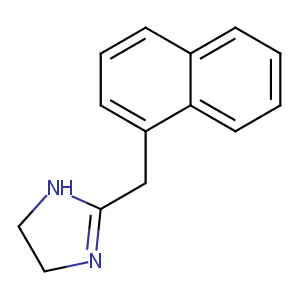 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 210.27 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.1 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Hyperaemia | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 9A61-9B7Y | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Naphazoline (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
|---|---|---|---|---|---|
| 2 | Toxnet: Naphazoline Hydrochloride | ||||
| 3 | Decongestants in treatment of nasal obstruction. Otolaryngol Pol. 1999;53(3):347-52. | ||||
| 4 | Cusson JR, Goldenberg E, Larochelle P "Effect of a novel monoamine-oxidase inhibitor, moclobemide on the sensitivity to intravenous tyramine and norepinephrine in humans." J Clin Pharmacol 31 (1991): 462-7. [PMID: 2050833] | ||||
| 5 | Product Information. Cymbalta (duloxetine). Lilly, Eli and Company, Indianapolis, IN. | ||||
