Details of the Drug
General Information of Drug (ID: DMJSDBE)
| Drug Name |
Barnidipine
|
|||||
|---|---|---|---|---|---|---|
| Synonyms |
Barnidipine [INN]; Barnidipine HCl; Barnidipino; Barnidipino [INN-Spanish]; Barnidipinum; Barnidipinum [INN-Latin]; Libradin; Mepirodipine; VXMOONUMYLCFJD-DHLKQENFSA-N; Vasexten (TN); YM 09730; barnidipine; (S)-3-((S)-1-Benzylpyrrolidin-3-yl) 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate; 104713-75-9; 2VBY96ASWJ; AC1L9FD3; BCP07244; C27H29N3O6; CHEBI:135793; CHEMBL2103761; CHEMBL2110040; CTK8B6661; SCHEMBL49302; UNII-2VBY96ASWJ
|
|||||
| Affected Organisms |
Humans and other mammals
|
|||||
| ATC Code | ||||||
| Structure |
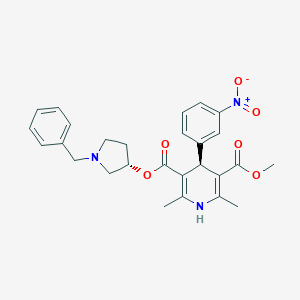 |
|||||
| 3D MOL | 2D MOL | |||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 491.5 | ||||
| Logarithm of the Partition Coefficient (xlogp) | 4.2 | |||||
| Rotatable Bond Count (rotbonds) | 8 | |||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | |||||
| Hydrogen Bond Acceptor Count (hbondacc) | 8 | |||||
| ADMET Property |
|
|||||
| Chemical Identifiers |
|
|||||
| Cross-matching ID | ||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
References
