Details of the Drug
General Information of Drug (ID: DMKI7RB)
| Drug Name |
Clomifene
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Androxal; Chlomaphene; Chloramifene; Cisclomifenum; Cisclomiphene; Clomifen; Clomifeno; Clomifenum; Clomifert; Clomiphene; Clostilbegit; Enclomifene; Enclomifeno; Enclomifenum; Enclomiphen; Enclomiphene; Klostilbegit; Transclomifenum; Transclomiphene; Zuclomifene; Zuclomifeno; Zuclomifenum; Zuclomiphene; Clomiphene B;Enclomiphene [USAN]; ISOMER B; Zuclomiphene [USAN]; Cis-Clomifene; Cis-Clomiphene; Clomid (TN); Clomifene (INN); Clomifene (TN); Clomifene [INN:BAN]; Clomifeno [INN-Spanish]; Clomifenum [INN-Latin]; En-Clomiphene; Enclomifeno [INN-Spanish]; Enclomifenum [INN-Latin]; Enclomiphene (USAN); Milophene (TN); RMI 16,289; RMI-16289; RMI-16312; Serophene (TN); Trans-Clomifene; Trans-Clomiphene; Zuclomifeno [INN-Spanish]; Zuclomifenum [INN-Latin]; Zuclomiphene (USAN); Cis-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; Trans-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; Cis-2-(4-(2-Chloro-1,2-diphenylethenyl)phenoxy)-N,N-diethylethanamine; Trans-2-(4-(2-Chloro-1,2-diphenylethenyl)phenoxy)-N,N-diethylethanamine; (E)-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; (Z)-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; (Z)-isomer; 1-(p-(beta-Diethylaminoethoxy)-phenyl)-1,2-diphenylchloroethylene; 2-(4-(2-Chloro-1,2-diphenylethenyl)phenoxy)-N,N-diethylethanamine; 2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; 2-(p-(beta-Chloro-alpha-phenylstyryl)phenoxy)-triethylamine; 2-(p-(beta-chloro-alpha-phenylstyryl)phenoxy)triethylamine; 2-({4-[(Z)-2-chloro-1,2-diphenylethenyl]phenyl}oxy)-N,N-diethylethanamine; 2-[4-(2-chloro-1,2-diphenylethenyl)phenoxy]-N,N-diethylethanamine; 2-[4-[(E)-2-chloro-1,2-diphenylethenyl]phenoxy]-N,N-diethylethanamine; 2-[4-[(Z)-2-chloro-1,2-diphenylethenyl]phenoxy]-N,N-diethylethanamine; 2-{4-[(E)-2-chloro-1,2-diphenylethenyl]phenoxy}-N,N-diethylethanamine
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Fertility Agents
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
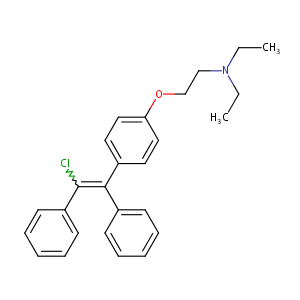 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 406 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7.2 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Female infertility | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | GA31.Z | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7619). | ||||
|---|---|---|---|---|---|
| 2 | [Stimulation of spermatogenesis: For whom? Why? How?]. Gynecol Obstet Fertil. 2016 Sep;44(9):505-16. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | The future of the new selective estrogen receptor modulators. Menopause Int. 2007 Mar;13(1):27-34. | ||||
| 7 | Antiestrogens and steroid hormones: substrates of the human P-glycoprotein. Biochem Pharmacol. 1994 Jul 19;48(2):287-92. | ||||
| 8 | CYP2D6 is primarily responsible for the metabolism of clomiphene. Drug Metab Pharmacokinet. 2008;23(2):101-5. | ||||
