Details of the Drug
General Information of Drug (ID: DMLKSE0)
| Drug Name |
Hexachlorophene
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acigena; Almederm; Armohex; Bilevon; Bivelon; Cotofilm; Dermadex; Distodin; Eleven; Esaclorofene; Exofene; Fascol; Fomac; Fostril; Gamophen; Gamophene; Hexabalm; Hexachlorofen; Hexachlorophane; Hexachlorophen; Hexachlorophenum; Hexaclorofeno; Hexafen; Hexascrub; Hexide; Hexophene; Hexosan; Isobac; Nabac; PHisoHex; Phisodan; Ritosept; Septisol; Septofen; Steral; Steraskin; Surofene; TRICHLOROPHENE; Tersaseptic; Trisophen; Turgex; Blockade Anti Bacterial Finish; Brevity Blue Liquid Bacteriostatic Scouring Cream; Brevity Blue Liquid Sanitizing Scouring Cream; Enditch Pet Shampoo; Esaclorofene [DCIT]; Fo stril; Hexachlorofen [Czech]; Hexachlorophene [INN]; Hilo Cat Flea Powder; Hilo Flea Powder; Neosept V; Pedigree Dog Shampoo Bar; Scrubteam Surgical Spongebrush; Staphene O; AT 7; B 32; H3P; Hexachlorophene [UN2875] [Poison]; KUC106447N; M0219; Nabac 25 ec; AT-7; AT17 (TN); AT7 (TN); Acigena (TN); Almederm (TN); At-17; B & b Flea Kontroller for Dogs Only; B 32 (VAN); Bilevon (TN); E-Z Scrub; En-Viron D Concentrated Phenolic Disinfectant; Exofene (TN); Fesia-sin; Fostril (TN); G-11; G-Eleven; G-II; Gamophen (TN); Germa-Medica; Hexa-Germ; Hexachlorophene, Pharma; Hexachlorophenum [INN-Latin]; Hexaclorofeno [INN-Spanish]; Hexaphene-LV; Hexosan (TN); PRE-OP II; Phiso-Scrub; Phisohex(TN); Pre-Op; Septi-Soft; Septisol (TN); Solu-Heks; Soy-dome; Ster-zac; Surgi-Cen; Surgi-Cin; Surofene (TN); Thera-Groom Pet Shampoo for Dogs for Veterinary Use Only; G-11 (TN); GERMA-MEDICA (MG); Germa-Medica (TN); Hexachlorophene (USP/INN); KSC-19-051; Methylenebis(3,4,6-trichlorophenol); Bis(2,3,5-tric hloro-6-hydroxyphenyl)methane; Bis(2,3,5-trichloro-6-hydroxyphenyl)methane; Bis(2-hydroxy-3,5,6-trichlorophenyl)methane; Bis(3,5,6-trichloro-2-hydroxyphenyl)methane; Bis-2,3,5-trichlor-6-hydroxyfenylmethan; Bis-2,3,5-trichloro-6-hydroxyfenylmethan; Bis-2,3,5-trichloro-6-hydroxyfenylmethan [Czech]; Bis(3,5,6-trichloro-2-hydroxyphenyl)-methane; Methane, bis(2,3,5-trichloro-6-hydroxyphenyl); 2,2',3,3',5,5'-Hexachloro-6,6'-dihydroxydiphenylmethane; 2,2'-Dihydroxy-3,5,6,3',5',6'-hexachlorodiphenylmethane; 2,2'-Methylene bis(3,4,6-trichlorophenol); 2,2'-Methylenebis(3,4,6-trichlorophenol); 2,2'-Methylenebis[3,4,6-trichlorophenol]; 2,2'-methanediylbis(3,4,6-trichlorophenol); 2,2-Methylene-bis-[3,4,6-trichlorophenol]; 3,3',5,5',6,6'-Hexachloro-2,2'-dihydroxydiphenylmethane; 3,4,6-trichloro-2-[(2,3,5-trichloro-6-hydroxyphenyl)methyl]phenol
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Antiinfective Agents
|
||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
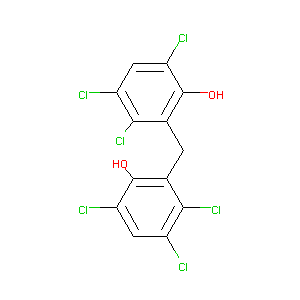 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 406.9 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 7.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
