Details of the Drug
General Information of Drug (ID: DMM1LG2)
| Drug Name |
Dihydroxyacetone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1,3-dihydroxyacetone; Dihydroxyacetone; 96-26-4; 1,3-Dihydroxypropan-2-one; Chromelin; 1,3-Dihydroxy-2-propanone; glycerone; Triulose; Viticolor; Soleal; Oxatone; Dihyxal; Oxantin; Otan; 2-Propanone, 1,3-dihydroxy-; 1,3-Dihydroxypropanone; 1,3-Dihydroxydimethyl ketone; NSC-24343; Ketochromin; Bis(hydroxymethyl) ketone; UNII-O10DDW6JOO; dihydroxy-acetone; 2-Propanone, 1,3-dihydroxy; BRN 1740268; CCRIS 4899; AI3-24477; Dihydroxyacetone [USP]; EINECS 202-494-5; O10DDW6JOO; 1,2-Dihydroxy-2-propanone; CHEBI:16016; RXKJFZQQPQGTFL-UHFFFAOYSA-N
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
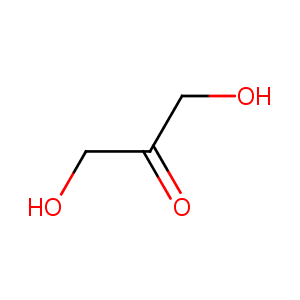 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 90.08 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1.4 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
