Details of the Drug
General Information of Drug (ID: DMP5A4T)
| Drug Name |
Trilaciclib
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
G1T28; 1374743-00-6; Trilaciclib [USAN]; G1T28(Trilaciclib); GTPL9626; CHEMBL3894860; SCHEMBL10082028; BDBM253928; US9464092, T; HY-101467; CS-0021431; 2'-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)amino)-7',8'-dihydro-6'H-spiro(cyclohexane-1,9'-pyrazino(1',2':1,5)pyrrolo(2,3-d)pyrimidin)-6'-one; Spiro(cyclohexane-1,9'(6'H)-pyrazino(1',2':1,5)pyrrolo(2,3-d)pyrimidin)-6'-one, 7',8'-dihydro-2'-((5-(4-methyl-1-piperazinyl)-2-pyridinyl)amino)-; 2-[[5-(4-methylpiperazin-1-yl)pyridin-2-yl]amino]spiro[7,8-dihydropyra
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||||||
| Structure |
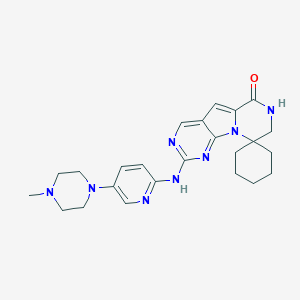 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 446.5 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.6 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Small-cell lung cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2C25.Y | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
