Details of the Drug
General Information of Drug (ID: DMP5YFQ)
| Drug Name |
4-methoxyphenylboronic acid
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
4-Methoxyphenylboronic acid; 5720-07-0; (4-Methoxyphenyl)boronic acid; 4-Methoxybenzeneboronic acid; 4-Methoxyphenyl boronic acid; p-Anisylboronic acid; p-Methoxyphenylboronic acid; 45713-46-0; p-Methoxybenzeneboronic acid; (4-Methoxyphenyl)Boranediol; Benzeneboronic acid, p-methoxy-; 4-Boronoanisole; Boronic acid, p-methoxyphenyl-; 4-methoxy boronic acid; BRN 2936912; CHEMBL21427; BORONIC ACID, (4-METHOXYPHENYL)-; 4-methoxy-phenyl-boronic acid; AI3-61385; (4-METHOXYPHENYL)BORANE; MFCD00039139; 4-Methoxyphenylboronic acid, 97%
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
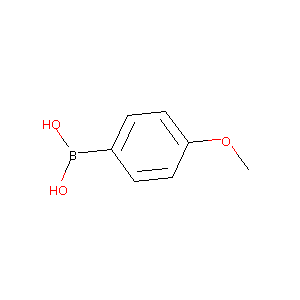 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 |
Molecular Weight | 151.96 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient | Not Available | ||||||||||||||||||||||
| Rotatable Bond Count | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
References
